Abstract
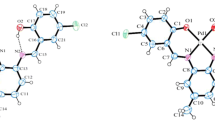
The title compound is a new pyrazolone derivative which was synthesized starting from p-sulphophenyl-3-methyl-5-pyrazolone (1) by nitrosation at low temperature to afford the corresponding p-sulphophenyl-3-methyl-4-nitroso-5-pyrazolone which can exist both in nitroso (2a) and oxime tautomeric forms (2b). Reduction of the latter using zinc with hydrochloric acid furnished the 4-amino-p-sulphophenyl-3-methyl-5-pyrazolone (3). The diazotization of (3) under careful control of temperature and pH afforded the p-sulphophenyl-3-methyl-5-pyrazolone diazonium salt (4) which was re-crystallized from acidified ethanol to afford crystal suitable for X-ray studies. UV–visible spectrum and cyclic voltammetric studies were also carried out indicating λmax at 420 nm and HOMO-LUMMO energy gap was also calculated (Eg) of 2.95 eV. The molecular and crystal structures of the compound were clarified by single crystal X-ray diffraction indicated that it crystallizes as the sodium salt in the triclinic space group P -1, with the 4-azo-pyrazolone and the sulphophenyl groups being nearly coplanar. To get an insight to the intermolecular interactions in the crystal, a Hirshfeld surface analysis was also carried out.
Graphical abstract















Similar content being viewed by others
Data availability
The crystal structure and characterization data are available.
Code availability
NA.
References
Roglans A, Pla-Quintana A, Moreno-Manas M (2006) Diazonium salts as substrates in palladium-catalyzed cross-coupling reactions. Chem Rev 106:4622–4643
Mahouche-Chergui S, Gam-Derouich S, Mangeney C, Chehimi MM (2011) Aryl diazonium salts: a new class of coupling agents for bonding polymers, biomacromolecules and nanoparticles to surfaces. Chem Soc Rev 40:4143–4166
Mo F, Dong G, Zhang Y, Wang J (2013) Recent applications of arene diazonium salts in organic synthesis. Org Biomol Chem 11:1582–1593
Meerwein H, Büchner E, van Emster K (1939) Über die Einwirkung aromatischer Diazoverbindungen auf α, β-ungesättigte Carbonylverbindungen. J Prakt Chem 152:237–266
Heinrich MR (2009) Intermolecular olefin functionalisation involving aryl radicals generated from arenediazonium salts. Chem-A Eur J 15:820–833
Galli C (1988) Radical reactions of arenediazonium ions: an easy entry into the chemistry of the aryl radical. Chem Rev 88:765–792
Hari DP, König B (2013) The photocatalyzed Meerwein arylation: classic reaction of aryl diazonium salts in a new light. Angew Chem Int Ed 52:4734–4743
Pratsch G, Anger CA, Ritter K, Heinrich MR (2011) Hydroxy-and aminophenyl radicals from arenediazonium salts. Chem-A Eur J 17:4104–4108
Heinrich MR, Blank O, Wölfel S (2006) Reductive carbodiazenylation of nonactivated olefins via aryl diazonium salts. Org Lett 8:3323–3325
Wetzel A, Pratsch G, Kolb R, Heinrich MR (2010) Radical arylation of phenols, phenyl ethers, and furans. Chem-A Eur J 16:2547–2556
Staples MK, Grange RL, Angus JA, Ziogas J, Tan NP, Taylor MK, Schiesser CH (2011) Tandem free-radical addition/substitution chemistry and its application to the preparation of novel AT 1 receptor antagonists. Organ Biomol Chem 9:473–479
Xiao T, Dong X, Tang Y, Zhou L (2012) Phenanthrene synthesis by eosin Y-catalyzed, visible light-induced [4+ 2] benzannulation of biaryldiazonium salts with alkynes. Adv Synth Catal 354:3195–3199
Brogden RN (1986) Pyrazolone derivatives. Drugs 32:60–70
Wang X, Wang X, Liang Y, Shi Z, Zhang J, Chen L, Fu L (2010) A cell-based screen for anticancer activity of 13 pyrazolone derivatives. Chin J Cancer 29:980–987
Vijesh AM, Isloor AM, Isloor S, Shivananda KN, Shyma PC, Arulmoli T (2011) Synthesis of some new pyrazolone derivatives as potent antimicrobial agents. Der Pharma Chemica 3:454–463
Chen T, Benmohamed R, Kim J, Smith K, Amante D, Morimoto RI, Kirsch DR, Ferrante RJ, Silverman RB (2012) ADME-guided design and synthesis of aryloxanyl pyrazolone derivatives to block mutant superoxide dismutase 1 (SOD1) cytotoxicity and protein aggregation: potential application for the treatment of amyotrophic lateral sclerosis. J Med Chem 55:515–527
Lalit K, Chandresh T, Vivek S (2012) Biological significance of pyrazolone: a review. Int J Res Pharm Sci 2:13–22
Soni JP, Sen DJ, Modh KM (2011) Structure activity relationship studies of synthesised pyrazolone derivatives of imidazole, benzimidazole and benztriazole moiety for anti-inflammatory activity. J Appl Pharm Sci 1:115–120
Antre RV, Oswal RJ, Kshirsagar SS, Kore PP, Mutha MM (2012) 2D-QSAR studies of substituted pyrazolone derivatives as anti-inflammatory agents. Med chem 2:126–130
Dohutia CHANDRAJIT, Kaishap PP, Chetia DIPAK (2013) Synthesis and study of analgesic, anti-inflammatory activities of 3-methyl-5-pyrazolone derivatives. Int J Pharm Pharm Sci 5:86–90
Antre RV, Cendilkumar A, Goli D, Andhale GS, Oswal RJ (2011) Microwave assisted synthesis of novel pyrazolone derivatives attached to a pyrimidine moiety and evaluation of their anti-inflammatory, analgesic and antipyretic activities. Saudi Pharm J 19:233–243
Bruker, SMART and SAINT, Bruker AXS Inc. 2002, Madison, Wisconsin, USA
Sheldrick GM (2004) SADABS. University of Göttingen, Germany
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A64:112–122
Farrugia LJ (2012) WinGX and ORTEP for Windows: an update. J Appl Crystallogr 45:849–854
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theoret Chim Acta 44:129–138
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis. CrystEngComm 2009(11):19–32
Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Spackman PR, Jayatilaka D, Spackman MA. CrystalExplorer17. 2017, The University of Western Australia
Venkatesan P, Thamotharan S, Ilangovan A, Liang H, Sundius T (2016) Crystal structure, Hirshfeld surfaces and DFT computation of NLO active (2E)-2-(ethoxycarbonyl)-3-[(1-methoxy-1-oxo-3-phenylpropan-2-yl) amino] prop-2-enoic acid. Spectrochim Acta Part A Mol Biomol Spectrosc 153:625–636
McKinnon JJ, Jayatilaka D, Spackman MA (2007) Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem Commun 37:3814–3816
Hartwar VR, Sist M, Jorgensen MRV, Mamakhel AH, Wang X, Hoffmann CM, Sugimoto K, Overgaard J, Iversen BB (2015) Quantitative analysis of intermolecular interactions in orthorhombic rubrene. IUCrJ 2:563–574
Funding
The authors are grateful to Quaid-I-Azam University, Islamabad, Pakistan for providing research funds.
Author information
Authors and Affiliations
Contributions
Ghulam Shabir: synthesis and interpretation, electrochemical studies. Ghulam Hussain: synthesis and data collection. Aamer Saeed: conceptualization, manuscript writing. Tasawwar Hussain: writing draft, optical studies. Tuncer Hökelek: Hirshfeld surface analysis. Mauricio F. Erben: optical studies. Ulrich Flörke: crystallography.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Research highlights
1. Solid stable diazonium salt, synthetic precursor towards acid Azo dyes
2. Triclinic geometry with coplanar 4-azo-pyrazolone and the sulphophenyl groups
3. Absorption maximum (λmax) in visible range at 420 nm
Rights and permissions
About this article
Cite this article
Shabir, G., Hussain, G., Saeed, A. et al. Investigation of stable solid diazonium salt by molecular structure, Hirshfeld surface analysis, optical and electrochemical studies, and applications. J Mol Model 27, 296 (2021). https://doi.org/10.1007/s00894-021-04910-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04910-1