Abstract
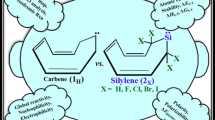
We compared and contrasted the ΔΕs-t, band gap (ΔΕHOMO-LUMO), aromaticity, charge distribution, and reactivity of singlet (s) and triplet (t) benzopyridine-4-ylidene as the fused remote N-heterocyclic carbene (frNHC) and frNHSis with different fused aromatic rings, at (U)B3LYP/AUG-cc-pVTZ and (U)M06-2X/AUG-cc-pVTZ levels of theory. In this investigation, we found (1) all s and t divalent states appear as minimum structures, for having no negative force constant. Nonetheless, only singlets present more thermodynamic stability than their triplet analogous; (2) the trend of ΔΕs-t in kcal/mol is ortho-pyrrole (52.94) > ortho-furan (51.84) > ortho-thiophene (50.38) > para-furan (49.36) > para-pyrrole (49.00) > para-phosphole (48.67) ≥ para-thiophene (48.64) > benzene (44.33) > ortho-phosphole frNHSi (27.50), while ΔΕs-t of frNHC is 15.65 kcal/mol; (3) apart from phosphole frNHSis, the order of ΔΕs-t in a “ortho position or zigzag array” about 1.8–4.0 kcal/mol is more than that of in a “para position or chair array”; (4) the highest ΔΕHOMO-LUMO is demonstrated by ortho-pyrrole frNHSi (95.65 kcal/mol) while the lowest ΔΕHOMO-LUMO is verified by the reference frNHC (63.44 kcal/mol); (5) in contradiction of frNHC, all singlet frNHSis reveal higher band gap and lower global reactivity than their triplet congeners; (6) charge distribution along with MEP maps indicate differentially electronic cloud in middle of rings frNHSis vs. frNHC; (7) we anticipate higher nucleophilicity and lower electrophilicity of triplet frNHSis than singlet congeners, will make them worthy of synthetic surveys.
Graphical abstract










Similar content being viewed by others
References
Kassaee MZ, Zandi H, Haerizade BN, Ghambarian M (2012) Effects of α-mono heteroatoms (N vs. P), and β-conjugation on cyclic silylenes. Comput Theor Chem 1001:39
Momeni MR, Shakib FA (2011) Theoretical Description of Triplet Silylenes Evolved from H2Si=Si. Organomet 30:5027
Ayoubi-Chianeh M, Kassaee MZ, Ashenagar S, Cummings PT (2019) Nucleophilicity of cyclic conjugated silylenes using DFT method. Phys Org Chem 32:ee3956
Brück A, Gallego D, Wang W, Irran E, Driess M, Hartwig JF (2012) Pushing the σ-donor strength in iridium pincer complexes: bis(silylene) and BIS(germylene) ligands are stronger donors than bis(phosphorus(III)) ligands. Angew Chem Int Ed 51:11478
Li J, Merkel S, Henn J, Meindl K, Döring A, Roesky HW, Ghadwal RS, Stalke D (2010) Lewis-base-stabilized dichlorosilylene: a two-electron σ-donor ligand. Inorg Chem 49:775
Yang W, Fu H, Wang H, Chen M, Ding Y, Roesky HW, Jana A (2009) A base-stabilized silylene with a tricoordinate silicon atom as a ligand for a metal complex. Inorg Chem 48:5058
Yamada T, Mawatari A, Tanabe M, Osakada K, Tanase T (2009) Planar tetranuclear and dumbbell-shaped octanuclear palladium complexes with bridging silylene ligands. Angew Chem 121:576
Blom B, Enthaler S, Inoue S, Irran E, Driess M (2013) Electron rich iron N-heterocyclic silylene complexes: synthesis, structure and catalytic activity. J Am Chem Soc 135:6703
Tan G, Blom B, Gallego D, Driess M (2014) Facile access to mono- and dinuclear heteroleptic N-heterocyclic silylene copper complexes. Organomet 33:363
Blom B, Stoelzel M, Driess M (2013) New vistas in N-heterocyclic silylene (NHSi) transition-metal coordination chemistry: syntheses, structures and reactivity towards activation of small molecules. Chem Eur J 19:40
Stoelzel M, Präsang C, Blom B, Driess M (2013) N-Heterocyclic Silylene (NHSi) Rhodium and iridium complexes: synthesis, structure, reactivity, and catalytic ability. Aust J Chem 66:1163
Protchenko AV, Birjkumar KH, Dange D, Schwarz AD, Vidovic D, Jones C, Kaltsoyannis N, Mountford P, Aldridge S (2012) A Stable Two-Coordinate Acyclic Silylene. J Am Chem Soc 134:6500
Rekken BD, Brown TM, Fettinger JC, Tuononen HM, Power PP (2012) Isolation of a stable, acyclic, two-coordinate silylene. J Am Chem Soc 134:6504
Asay M, Inoue S, Driess M (2011) Aromatic ylide-stabilized carbocyclic silylene, angew. Chemie Int Ed 50:9589
Sasamori T, Tokitoh N (2005) In: King RB (ed) Encyclopedia of inorganic chemistry II. Wiley, Chichester, p 1698
Slipchenko LV, Krylov AI (2002) Singlet-triplet gaps in diradicals by the spin-flip approach: a benchmark study. J Chem Phys 117:4694
Denk M, Lennon R, Hayashi R, West R, Haaland A, Belyakov H, Verne P, Wagner M, Metzler N (1994) Synthesis and structure of a stable silylene. J Am Chem Soc 116(6):2691
B. Gehrhus, M.F. Lappert, J. Heinicke, R. Boese, D. Bläser, Synthesis, structures and reactions of new thermally stable silylenes, J. Chem. Soc. Chem. Commun. 0 (1995) 1931.
West R, Denk M (1996) Stable silylenes: synthesis, structure, reactions. Pure Appl Chem 68(4):785
Heinicke J, Oprea A, Kindermann MK, Karpati T, Nyulaszi L, Veszpremi T, Hitchcock PB, Lappert MF, Maciejewski H (1998) Silylenenickel(0) or Silyl(silylene)platinum(II) Complexes by reaction of Si[(NCH2But)2C6H4-1,2] with [NiCl2(PPh3)2], [Ni(cod)2], or [PtCl2(PPh3)2]. Organomet 17:5599
Kira M, Ishida S, Iwamoto T, Kabuto C (1999) The First Isolable Dialkylsilylene. J Am Chem Soc 121:9722
Driess M, Yao S, Brym M, Wüllen CV, Lentz D (2006) A new type of N-heterocyclic silylene with ambivalent reactivity. J Am Chem Soc 128(30):9628
Koohi M, Bastami H (2020) Substituted Hammick carbenes: The effects of fused rings and hetero atoms through DFT calculations. J Phys Org Chem 33:e4023
Söğütlü İ, Soltanzadeh M, Mert H, Mert N, Vessally E (2021) Substituent effects on the stability of cyclic - unsaturated remote N-heterocyclic Hammick carbenes using density functional theory. J Mol Struct 1230:129821
Zhao H, Yang D, Zhou Y, Fang Y, Shi M, Vessally E (2020) A computational quest for the effects of fused rings on the stability of Hammick carbenes type remote N-heterocyclic carbenes. J Chin Chem Soc 68:76
Zhao K, Zhang Y, Ma Y, Jin Z, Rashid Sheykhahmad F (2020) Stabilization of novel N-heterocyclic germylenes (NHGes): a computational perspective. J Chin Chem Soc. https://doi.org/10.1002/jccs.202000296
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14(11):1347
Sobolewski AL, Domcke W (2002) Ab initio investigation of the structure and spectroscopy of hydronium−water clusters. J Phys Chem A 106:4158
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098
Becke ADJ (1993) Density-functional thermochemistry. III. The role of exact exchange. Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Account 120:215
Hariharan PC, Pople JA (1974) Accuracy of AH, equilibrium geometries by single determinant molecular orbital theory. Mol Phys 27:209
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second row elements. J Chem Phys 77:3654
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PVR (1983) Efficient diffuse function-augmented basis sets for Anion Calculations. III. The 3–21+G set for first-row elements, Li-F. J Comput Chem 4:294
Frisch MJ, Pople JA, Binkley JSJ (1984) Self-consistent molecular orbital methods 25: Supplementary Functions for Gaussian Basis Sets. Chem Phys 80:3265
Kendall RA, Dunning TH Jr, Harrison RJJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. Chem Phys 96:6796
Krishna R, Frisch MJ, Pople JA (1980) Contribution of triple substitutions to the electron correlation energy in fourth order perturbation theory. J Chem Phys 72:4244
Hehre WJ, Radom L, Schleyer PVR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Foresman JB, Frisch A (1996) Exploring chemistry with electronic structure methods. Gaussian Inc, Pittsburgh
Weinhold F, Glendening ED. NBO Version 7.0 program manual natural bond orbital analysis programs
Weinhold F (2012) Natural bond orbital analysis: a critical overview of relationships to alternative bonding perspectives. J Comput Chem 33:2363
Glendening ED, Landis CR, Weinhold F (2012) Natural bond orbital methods. Wiley Interdiscip Rev Comput Mol Sci 2:1
Zhang G, Musgrave CB (2007) Comparison of DFT methods for molecular orbital eigenvalue calculations. J Phys Chem A 111:1554
Schleyer PVR, Maerker C, Dransfeld A, Jiao H, Hommes NJRVE (1996) Nucleus-independent chemical shifts (NICS): a simple and efficient aromaticity probe. J Am Chem Soc 118:6317
Schleyer PVR, Jiao H, Hommes NJRVE, Malkin VG, Malkina OL (1997) An evolution of the aromaticity of inorganic rings: refined evidence from magnetic properties. J Am Chem Soc. 119:12669
Schleyer PVR, Manoharan M, Wang Z, Kiran B, Jiao H, Puchta R, van EikemaHommes NJR (2001) Dissected nucleus-independent chemical shift analysis of p-aromaticity and antiaromaticity. Org Lett 3(16):2465
Domingo LR, Chamorro E, Pérez PJ (2008) Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions : a theoretical study. Org Chem 73:4615
Parr RG, Szentpaly L, Liu S (1999) Electrophilicity Index. J Am Chem Soc 121:1922
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Zhang H, Sun M, Song L, Guo J, Zhang L (2019) Fate of NaClO and membrane foulants during in-situ cleaning of membrane bioreactors: combined effect on thermodynamic properties of sludge. Biochem Eng J 147:146
Sun M, Yan L, Zhang L, Song L, Guo J, Zhang H (2019) New insights into the rapid formation of initial membrane fouling after in-situ cleaning in a membrane bioreactor. Process Biochem 78:108
Min Yanga CLLL (2021) Predictive model of convective heat transfer coefficient in bone micro-grinding using nanofluid aerosol cooling. Int Commun Heat Mass Transfer 125:105317
Huang B, Li C, Zhang Y, Ding W, Yang M, Yang Y, Zhai H, Xu X, Wang DS, Debnath M, Jamil H, NanLi H, Ali M, Gupta MK, Said Z (2021) Advances in fabrication of ceramic corundum abrasives based on sol–gel process. Chin J Aeronaut 34:1
Zhang J, Wu W, Li C, Yang M, Zhang Y, Jia D, Hou Y, Li R, Cao H, Ali HM (2021) Convective heat transfer coefficient model under nanofluid minimum quantity lubrication coupled with cryogenic air grinding Ti–6Al–4V. Int J Precis Eng Manuf Green Technol 8:1113
Duan Z, Li C, Ding W, Zhang Y, Yang M, Gao T, Cao H, Xu X, Wang D, Mao C, Li HN, Kumar GM, Said Z, Debnath S, Jamil M, Ali HM (2021) Milling force model for aviation aluminum alloy: academic insight and perspective analysis. Chinese J Mech Eng 34(1):1
Dai Z, Xie J, Fan X, Ding X, Liu W, Zhou S, Ren X (2020) Enhanced energy storage properties and stability of Sr(Sc0.5Nb0.5)O3 modified 0.65BaTiO3-0.35Bi0.5Na0.5TiO3 ceramics. Chem Eng J (Lausanne, Switzerland: 1996) 397:125520
Wang Q, Sun S, Zhang X, Liu H, Sun B, Guo S (2021) Influence of air oxidative and non-oxidative torrefaction on the chemical properties of corn stalk. Bioresour Technol 332:125120
Li X, Shi T, Li B, Chen X, Zhang C, Guo Z, Zhang Q (2019) Subtractive manufacturing of stable hierarchical micro-nano structures on AA5052 sheet with enhanced water repellence and durable corrosion resistance. Mater Des 183:108152
Duan Y, Liu Y, Chen Z, Liu D, Yu E, Zhang X, Fu H, Fu J, Zhang J, Du H (2020) Amorphous molybdenum sulfide nanocatalysts simultaneously realizing efficient upgrading of residue and synergistic synthesis of 2D MoS2 nanosheets/carbon hierarchical structures. Green Chem 22(1):44
Fan Z, Ji P, Zhang J, Segets D, Chen D, Chen S (2021) Wavelet neural network modeling for the retention efficiency of sub-15 nm nanoparticles in ultraltration under small particle to pore diameter ratio. J Membr Sci 635:119503
Luo G, Siong Teh K, Xia Y, Luo Y, Li Z, Wang S, Jiang Z (2019) A novel three-dimensional spiral CoNi LDHs on Au@ErGO wire for high performance fiber supercapacitor electrodes. Mater Lett 236:728–731
Zhang K, Qiu L, Tao J, Zhong X, Lin Z, Wang R, Liu Z (2021).Recovery of gallium from leach solutions of zinc refinery residues by stepwise solvent extraction with N235 and Cyanex 272. Hydrometallurgy 205:105722. https://doi.org/10.1016/j.hydromet.2021.105722
Zhang X, Zhang Y (2021) Experimental study on enhanced heat transfer and flow performance of magnetic nanofluids under alternating magnetic field. Int J Therm Sci 164:106897
Zhang X, Zhang Y (2021) Heat transfer and flow characteristics of Fe3O4 -water nanofluids under magnetic excitation. Int J Therm Sci 163:106826
Chen Z, Zhang H, He X, Fan G, Li X, He Z, Zhang L (2021) Fabrication of cellulosic paper containing zeolitic imidazolate framework and its application in removal of anionic dye from aqueous solution. BioResources 16(2):2644
Zhang L, Zheng J, Tian S, Zhang H, Guan X, Zhu S, Zhang X, Bai Y, Xu P, Zhang J, Li Z (2020) Effects of Al3+ on the microstructure and bioflocculation of anoxic sludge. J Environ Sci (China) 91:212
Zhang M, Zhang L, Tian S, Zhang X, Guo J, Guan X, Xu P (2020) Effects of graphite particles/Fe3+ on the properties of anoxic activated sludge. Chemosphere (Oxford) 253:126638
Zhang L, Wang H, Zhang X, Tang Y (2021) A review of emerging dual-ion batteries: fundamentals and recent advances. Adv Funct Mater 31(20):2010958
Pan Q, Zheng Y, Tong Z, Shi L, Tang Y (2021) Novel lamellar tetrapotassium pyromellitic organic for robust high-capacity potassium storage. Angew Chem (Int Ed) 60(21):11835
Tong X, Ou X, Wu N, Wang H, Li J, Tan Y (2021) High oxidation potential ≈60 V of concentrated electrolyte toward high-performance dual-ion battery. Adv Energy Mater 2100151. https://doi.org/10.1002/aenm.202100151
Yang K, Liu Q, Zheng Y, Yin H, Zhang S, Tang Y (2021) Locally ordered graphitized carbon cathodes for high-capacity dual-ion batteries. Angew Chem (Int Ed) 60(12):6326
Li Z, Cheng R, Chen F, Lin X, Yao X, Liang B, Huang C, Sun K, Wang A-J (2021) Selective stress of antibiotics on microbial denitrification: inhibitory effects, dynamics of microbial community structure and function. J Hazard Mater 405:124366
Boehme C, Frenking G (1996) Electronic structure of stable carbenes, silylenes, and germylenes. J Am Chem Soc 118:2039
Jursic BS (1999) Hybrid density functional theory study of low reactivity of imidazol-2-ylidine toward insertion and addition reactions. J Chem Soc Perkin Trans 2(8):1805
Author information
Authors and Affiliations
Contributions
We confirm that all the authors are aware of and approve of the submission (including the approval from the authorization or institution). We ensure that the order of authors you have entered in the system are same arrange/order with the names in the manuscript text.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vessally, E., Ebadi, A.G., Heravi, M.R.P. et al. Investigation of fused remote N-heterocyclic silylenes (frNHSis), at DFT. J Mol Model 27, 299 (2021). https://doi.org/10.1007/s00894-021-04899-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04899-7