Abstract
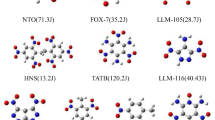
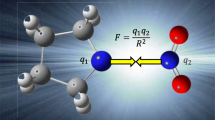
The dependence of sensitivity of an explosive on its molecular structure may be mainly attributed to the molecular deformability, which can be expressed by some characteristic parameters, resonance energy for aromatic an explosive, strain energy for a strained-ring or strained-cage explosive, large π-π separation energy for a large π-π linked-explosive, bond rotational energy barriers of C–NO2, N–NO2, O–NO2 for C–NO2, N–NO2, O–NO2 bond-based explosives, and so on. Molecular polarizability of an explosive is also an important molecular deformability index, which can be effectively used to compare impact sensitivities of explosive’s isomers, isoelectronic species, and similar structures. Interestingly, comparing the molecular polarizabilities under external electric fields with different energy levels of isomeric N20(Ih) and N20(D3d) clusters and the Mo2N20 and Re2N20 complex compounds, it is found that there are different energy thresholds of significant molecular expansion.










Similar content being viewed by others
References
Politzer P, Murray JS (2016). Prop. Explos. Pyrotech 41:414–425
Tan B, Peng R, Long X, Li H, Jin B, Chu S (2012). J. Mol. Model. 18:583–589
Tan B, Huang M, Huang H, Long X, Li J, Nie F, Huang J (2013). Prop. Explos. Pyrotech 38:372–378
Tan B, Huang M, Long X, Li J, Yuan X, Xu R (2014). Polyhedron 79:124–128
Tan B, Huang M, Long X, Li J, Yuan X, Xu R (2015). Int. J. Quantum Chem. 115:84–89
Tan B, Long X, Li J (2012). Comput. Theor. Chem. 993:66–72
Tan B, Li H, Huang H, Han Y, Li J, Li M, Long X (2019). Chem. Phys. 520:81–87
Tan B, Long X, Li J, Nie F, Huang J (2012). J. Mol. Model. 18:5127–5132
Tan B, Huang M, Li J, Long X (2016). Chin. Energ. Mater 24:10–18
Liu FC (1998). Chin. J. Light Scatt. 10:168–171
Ren FD, Cao DL, Shi WJ, You M (2017). RSC Adv. 7:47063–47072
Wei Y, Wang XQ, Wang X, Tao ZQ, Cui YQ, Yang ML (2016). RSC Adv. 6:24712–24718
Liu Y, Ma Y, Yu T, Lai W, Guo W, Ge Z, Ma Z (2018). J. Phys. Chem. A 122:2129–2134
Zhang G (1990). J. High Press. Phys. 4:161–166
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2010). J. Mol. Model. 16:895–901
Zhao K (2008) Dielectric spectroscopy methods and their applications1st edn. China Chemical Industry Press, Beijing
Tan B, Tan K, Li H, Yu W, Han Y, Li M, Long X (2018). Polyhedron 156:54–57
Miller KJ (1990). J. Am. Soc. 112:8533–8542
Jin P, Brinck T, Murray JS, Politzer P (2003). Int. J. Quantum Chem. 95:632–637
Pandey PKK, Santry DP (1980). J. Chem. Phys. 73:2899–2901
Cohen MJ, Willetts A, Amos RD, Handy NC (1994). J. Chem. Phys. 100:4467–4476
Perdew JP, Burke K, Ernzerhof M (1996). Phys. Rev. Lett. 77:3865–3868
Amado C, Barone V (1999). J. Chem. Phys. 110:6158–6169
Dunning Jr TH (1989). J. Chem. Phys. 90:1007–1023
Kendall RA, Dunning Jr TH, Harrison RJ (1992). J. Chem. Phys. 96:6796–6806
Cohen HD, Roothaan CCJ (1965). J. Chem. Phys. 43:S34–S39
Calaminici P, Jug K, Köster AM (1998). J. Chem. Phys. 109:7756
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas CO, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision D.01. Gaussian, Inc., Wallingford
Politzer P, Murray JS, Bulat FA (2010). J. Mol. Model. 16:1731–1742
Lu T, Chen FJ (2012). J. Comput. Chem. 33:580–592
Wheeler SE, Houk KN, Schleyer PR, Allen WD (2009). J. Am. Chem. Soc. 131:2547–2560
Møller C, Plesset MS (1934). Phys. Rev. 46:618
Inagaki S (2009) Orbitals in inorganic chemistry: metal ring and clusters, hydronitrogens and heterocycles. Top. Curr. Chem. 289:293–315
Gimarc BM, Zhao M (1996). Inorg. Chem. 35:3289–3297
Meyer R, Köhler J, Homburg A (2015) Explosives7th edn. WILEY-VCH VerlagGmbH & Co. KGaA, Weinheim
Koch EC, Klapötke TM (2012). Prop. Explos. Pyrotech 37:335–344
Zhang C (2009). J. Hazard. Mater. 161:21–28
Storm CB, Stine JR, Kramer JF (1990) In: Bulusu SN (ed) Chemistry and physics of energetic materials, ch 27. Kluwer, Dordrecht, pp 605–639
Sikder AK, Maddala G, Agrawal JP, Singh H (2001). J. Hazard. Mater. A 84:1–26
Л. И. ХМеЛъНИЦКИЙ, С. С. НоВИКОВ, Т. И. ГоЦоВИКоВа. (2013) Chemistry of Furazan (Russian edition), translated into Chinese by Yuanmjie Shu and Bozhou Wang, Chengdu Times Press, Chengdu, Chapter 1, pp 1–57
Tan B, Huang H, Huang M, Long X, Li J, Yuan X, Xu R (2014). J. Fluor. Chem. 158:29–3730
Liddle ST (2015) Molecular metal–metal bonds-compounds, synthesis, properties. Wiley-VCH Verlag GmbH & Co, Weinheim
Cotton FA, Murillo CA, Walton RA (2005) Multiple bonds between metal atoms. Springer Science and Business Media, Inc., Berlin
Hay PJ, Wadt WR (1985). J. Chem. Phys. 82:270–283
Wadt WR, Hay PJ (1985). J. Chem. Phys. 82:284–298
Hay PJ, Wadt WR (1985). J. Chem. Phys. 82:299–310
Funding
The authors acknowledge financial support from National Natural Science Foundation of China (11372289) and the Innovation Project of Development Foundations Supported by China Academy Engineering Physics (cx2019009). Some computations were performed at the Institute of Computational Science, CAEP.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tan, B., Chai, C., Tan, K. et al. Molecular polarizabilities of some energetic compounds. J Mol Model 27, 51 (2021). https://doi.org/10.1007/s00894-020-04540-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04540-z