Abstract
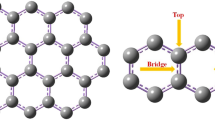
Adsorption behavior of nitrous oxide (N2O) on pristine graphene (PG) and tetracyanoethylene (TCNE) modified PG surfaces is investigated using density functional theory. A number of initial adsorbate geometries are considered on both surfaces and the most stable ones are chosen upon calculation of the adsorption energies (Eads). N2O is found to adsorb in a weakly exoergic process (Eads ∼ −3.18 kJ mol−1) at the equilibrium distance of 3.52 Å on the PG surface. N2O adsorption can be greatly enhanced with the presence of a TCNE molecule (Eads = −87.00 kJ mol−1). Mulliken charge analysis confirms that adsorption of N2O is not accompanied by distinct charge transfer from the surfaces to the molecule (˂ 0.001 │e│ for each case). Moreover, on the basis of calculated changes in the HOMO/LUMO energy gap, it is found that electronic properties of PG and TCNE modified PG are not sensitive toward adsorption of N2O, indicating that both surfaces are not good enough to introduce as an N2O detector. However, the considerable amount of Eads in TCNE modified PG can be a guide to the design of graphene-based adsorbents for N2O capture.




Similar content being viewed by others
References
Duce RA, LaRoche J, Altieri K, Arrigo KR, Baker AR, Capone DG, Cornell S, Dentener F, Galloway J, Ganeshram RS, Geider RJ, Jickells T, Kuypers MM, Langlois R, Liss PS, Liu SM, Middelburg JJ, Moore CM, Nickovic S, Oschlies A, Pedersen T, Prospero J, Schlitzer R, Seitzinger S, Sorensen LL, Uematsu M, Ulloa O, Voss M, Ward B, Zamora L (2008) Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science 320(5878):893–897. https://doi.org/10.1126/science.1150369
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N<sub>2</sub>O): the dominant ozone-depleting substance emitted in the 21st century. Science 326(5949):123–125. https://doi.org/10.1126/science.1176985
Reid CR, Thomas KM (1999) Adsorption of gases on a carbon molecular sieve used for air separation: linear adsorptives as probes for kinetic selectivity. Langmuir 15(9):3206–3218. https://doi.org/10.1021/la981289p
Saha D, Deng S (2010) Adsorption equilibrium and kinetics of CO2, CH4, N2O, and NH3 on ordered mesoporous carbon. J Colloid Interface Sci 345(2):402–409. https://doi.org/10.1016/j.jcis.2010.01.076
Yoosefian M (2017) Powerful greenhouse gas nitrous oxide adsorption onto intrinsic and Pd doped single walled carbon nanotube. Appl Surf Sci 392:225–230. https://doi.org/10.1016/j.apsusc.2016.09.051
Vermisoglou EC, Romanos GE, Karanikolos GN, Kanellopoulos NK (2011) Catalytic NOx removal by single-wall carbon nanotube-supported Rh nanoparticles. J Hazard Mater 194:144–155. https://doi.org/10.1016/j.jhazmat.2011.07.078
Rastegar SF, Peyghan AA, Hadipour NL (2013) Response of Si- and Al-doped graphenes toward HCN: a computational study. Appl Surf Sci 265(Supplement C):412–417. https://doi.org/10.1016/j.apsusc.2012.11.021
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306(5696):666–669. https://doi.org/10.1126/science.1102896
Rastegar SF, Hadipour NL, Tabar MB, Soleymanabadi H (2013) DFT studies of acrolein molecule adsorption on pristine and Al-doped graphenes. J Mol Model 19(9):3733–3740. https://doi.org/10.1007/s00894-013-1898-5
Pumera M, Scipioni R, Iwai H, Ohno T, Miyahara Y, Boero M (2009) A mechanism of adsorption of β-nicotinamide adenine dinucleotide on graphene sheets: experiment and theory. Chem Eur J 15(41):10851–10856
Yavari F, Koratkar N (2012) Graphene-based chemical sensors. J Phys Chem Lett 3(13):1746–1753
Ghiaci M, Ghazaie M (2016) Modification of a heterogeneous catalyst: sulfonated graphene oxide coated by SiO2 as an efficient catalyst for Beckmann rearrangement. Catal Commun 87:70–73. https://doi.org/10.1016/j.catcom.2016.09.007
Wang Y, Zhang W, Luo C, Wu X, Wang Q, Chen W, Li J (2016) Synthesis, characterization and enhanced electromagnetic properties of NiFe2O4@SiO2-decorated reduced graphene oxide nanosheets. Ceram Int 42(15):17374–17381. https://doi.org/10.1016/j.ceramint.2016.08.036
Chen J-H, Jang C, Adam S, Fuhrer M, Williams E, Ishigami M (2008) Charged-impurity scattering in graphene. Nat. Phys. 4(5):377–381
Chen W, Chen S, Qi DC, Gao XY, Wee ATS (2007) Surface transfer p-type doping of epitaxial graphene. J Am Chem Soc 129(34):10418–10422
Lu Y, Chen W, Feng Y, He P (2008) Tuning the electronic structure of graphene by an organic molecule. J Phys Chem B 113(1):2–5
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S (1993) General atomic and molecular electronic structure system. J Comput Chem 14(11):1347–1363
Friesner RA (2005) Ab initio quantum chemistry: methodology and applications. Proc Natl Acad Sci USA 102(19):6648–6653
Xu X, Goddard WA (2004) The X3LYP extended density functional for accurate descriptions of nonbond interactions, spin states, and thermochemical properties. Proc Natl Acad Sci USA 101(9):2673–2677
Peterson C, Penchoff DA, Wilson AK (2015) Ab initio approaches for the determination of heavy element energetics: ionization energies of trivalent lanthanides (ln= la-Eu). J Chem Phys 143(19):194109
Mulliken RS (1955) Electronic population analysis on LCAO–MO molecular wave functions. I. J Chem Phys 23(10):1833–1840
Xiao J, Sitamraju S, Janik MJ (2014) CO2 adsorption thermodynamics over N-substituted/grafted graphanes: a DFT study. Langmuir 30(7):1837–1844
Rastegar SF, Hadipour NL, Soleymanabadi H (2014) Theoretical investigation on the selective detection of SO2 molecule by AlN nanosheets. J Mol Model 20(9):2439. https://doi.org/10.1007/s00894-014-2439-6
Samadizadeh M, Rastegar SF, Peyghan AA (2015) The electronic response of nano-sized tube of BeO to CO molecule: a density functional study. Struct Chem 26(3):809–814. https://doi.org/10.1007/s11224-014-0548-6
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rastegar, S.F., Osouleddini, N. TCNE-modified graphene as an adsorbent for N2O molecule: a DFT study. J Mol Model 23, 352 (2017). https://doi.org/10.1007/s00894-017-3526-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3526-2