Abstract
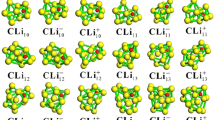
A systematic theoretical investigation on cobalt lithium clusters LinCo [1–12] was performed with a DFT approach. The location of global minima and structural evolution were carried out using the partical swarm optimization method. Li6Co is the transition structure in going from low-coordinated structures to three-dimensional torispherical structures with a cobalt atom enclosed by lithium atoms. Maxima of ∆2 E and E b for LinCo were found at n = 3, 6, 8, 10, indicating that these clusters possess higher relative stability than their neighbors. In comparison with small clusters, n = 1–6, the greater electron transfer from Li-2s to Co-3d within cage-like clusters LinCo (n = 7–12) strengthens the bonding effect between Lin and Co, which is reflected in the Wiberg bond index of Co and atomic binding energy analysis. AdNDP analysis verified the presence of both Lewis bonding elements (1c–2e objects) and delocalized bonding elements (6c–2e, 9c–2e and 10c–2e bonds). It is hoped that this theoretical work will provide favorable information to help understand the influence of dopant transition metal atoms on the properties of lithium-based materials.





Similar content being viewed by others
References
Nishijima M, Kagohashi T, Imanishi M, Takeda Y, Yamamoto O, Kondo S (1996) Synthesis and electrochemical studies of a new anode material, Li3-Xcoxn. Solid State Ionics 83:107–111
Stoeva Z, Smith RI, Gregory DH (2006) Stoichiometry and defect structure control in the ternary lithium nitridometalates Li3-X-Ynixn. Chem Mater 18:313–320
Shodai T, Okada S, Tobishima S, Yamaki J (1996) Study of Li(3-X)M(X)N (M:Co, Ni or Cu) system for use as anode material in lithium rechargeable cells. Solid State Ionics 86–8:785–789
Rowsell JLC, Pralong V, Nazar LF (2001) Layered lithium iron nitride: a promising anode material for Li-Ion batteries. J Am Chem Soc 123:8598–8599
Cabana J, Ionica-Bousquet CM, Grey CP, Palacin MR (2010) High rate performance of lithium manganese nitride and oxynitride as negative electrodes in lithium batteries. Electrochem Commun 12:315–318
Parry IS, Kartouzian A, Hamilton SM, Balaj OP, Beyer MK, Mackenzie SR (2015) Chemical reactivity on gas-phase metal clusters driven by blackbody infrared radiation. Angew Chem Int Ed 54:1357–1360
Moses MJ, Fettinger JC, Eichhorn BW (2003) Interpenetrating as-20 fullerene and Ni-12 icosahedra in the onion-skin [as@Ni-12@as-20](3-) Ion. Science 300:778–780
Kelty SP, Chen CC, Lieber CM (1991) Superconductivity at 30-K in cesium-doped C60. Nature 352:223–225
Yano J et al (2006) Where water is oxidized to dioxygen: structure of the photosynthetic Mn4ca cluster. Science 314:821–825
Yano J, Yachandra V (2014) Mn4ca cluster in photosynthesis: where and how water is oxidized to dioxygen. Chem Rev 114:4175–4205
Bethune DS, Johnson RD, Salem JR, Devries MS, Yannoni CS (1993) Atoms in carbon cages - the structure and properties of endohedral fullerenes. Nature 366:123–128
Jones RO, Lichtenstein A, Hutter J (1997) Density functional study of structure and bonding in lithium clusters Li-N and their oxides lino. J Chem Phys 106:4566–4574
Kudo H, Wu CH, Ihle HR (1978) Mass-spectrometric study of vaporization of Li2o(S) and thermochemistry of gaseous Lio, Li2o, Li3o, and Li2o2. J Nucl Mater 78:380–389
Deshpande M, Dhavale A, Zope RR, Chacko S, Kanhere DG (2000) Ground-State geometries and stability of impurity doped clusters: Linbe and Linmg (N=1–12). Phys Rev A 62
Li Y, Liu YJ, Wu D, Li ZR (2009) Evolution of the structures and stabilities of boron-doped lithium cluster cations: Ab initio and Dft studies. Phys Chem Chem Phys 11:5703–5710
Li Y, Wu D, Li ZR, Sun CC (2007) Structural and electronic properties of boron-doped lithium clusters: ab initio and DFT studies. J Comput Chem 28:1677–1684
Tong J, Li Y, Wu D, Li ZR, Huang XR (2009) Low ionization potentials of binuclear superalkali B2li11. J Chem Phys 131
Tai TB, Nguyen MT (2010) The high stability of boron-doped lithium clusters Li5b, Li6b+/− and Li7b: a case of the phenomenological shell model. Chem Phys Lett 489:75–80
Shah V, Kanhere DG (1996) Ground-state geometries and the stability of some Linal(M) clusters investigated using density-based Ab initio molecular dynamics. J Phys Condens Matter 8:L253–L260
Lievens P, Thoen P, Bouckaert S, Bouwen W, Vanhoutte F, Weidele H, Silverans RE, Navarro-Vazquez A, Schleyer PV (1999) Ionization potentials of hypervalent Linc (2 <= N <= 10). Eur Phys J D 9:289–295
Joshi K, Kanhere DG (2002) Ab initio investigation of electronic structure, equilibrium geometries, and finite-temperature behavior of Sn-Doped Li-N clusters. Phys Rev A 65:043203
Ngan VT, Gruene P, Claes P, Janssens E, Fielicke A, Nguyen MT, Lievens P (2010) Disparate effects of Cu and V on structures of exohedral transition metal-doped silicon clusters: a combined far-infrared spectroscopic and computational study. J Am Chem Soc 132:15589–15602
Ngan VT, De Haeck J, Le HT, Gopakumar G, Lievens P, Nguyen MT (2009) Experimental detection and theoretical characterization of germanium-doped lithium clusters linge (N=1–7). J Phys Chem A 113:9080–9091
Lievens P, Thoen P, Bouckaert S, Bouwen W, Vanhoutte F, Weidele H, Silverans RE, Navarro-Vazquez A, Schleyer PV (1999) Ionization potentials of lino (2 <= N <= 70) clusters: experiment and theory. J Chem Phys 110:10316–10329
Tai TB, Nguyen MT (2012) Electronic structure and thermochemical properties of silicon-doped lithium clusters Linsi0/+, N=1–8: new insights on their stability. J Comput Chem 33:800–809
Tighezza A, Rehspringer JL, Kappler JP, Drillon M (1993) Evidence of superconductivity at 30-K in Pr2cuo4-Xfx. Solid State Commun 86:59–62
Knight WD, Clemenger K, Deheer WA, Saunders WA, Chou MY, Cohen ML (1984) Electronic shell structure and abundances of sodium clusters. Phys Rev Lett 52:2141–2143
Velickovic SR, Koteski VJ, Cavor JNB, Djordjevic VR, Cveticanin JM, Djustebek JB, Veljkovic MV, Neskovic OM (2007) Experimental and theoretical investigation of new hypervalent molecules Linf (N=2–4). Chem Phys Lett 448:151–155
Velickovic S, Djordjevic V, Cveticanin J, Djustebek J, Veljkovic M, Neskovic O (2006) Ionization energies of Linx(N=2,3; X=Cl, Br, I) molecules. Rapid Commun Mass Spectrom 20:3151–3153
Senturk S (2011) A density functional study of Lincl (N=1–7) clusters. Z Naturforsch A 66:372–376
Velickovic SR, Dustebek JB, Veljkovic FM, Veljkovic MV (2012) Formation of positive cluster ions Linbr (N=2–7) and ionization energies studied by thermal ionization mass spectrometry. J Mass Spectrom 47:627–631
Senturk S, Unal A, Kalfa OM (2013) Density functional study of bromine-doped lithium clusters. Comput Theor Chem 1023:46–50
Dustebek J, Milovanovic M, Jerosimic S, Veljkovic M, Velickovic S (2013) Theoretical and experimental study of the non-stoichiometric lini (N=3 and 5) clusters. Chem Phys Lett 556:380–385
Zhang M, Zhang JF, Feng XJ, Zhang HY, Zhao LX, Luo YH, Cao W (2013) Magnetic superatoms in Vlin (N=1–13) clusters: a first-principles prediction. J Phys Chem A 117:13025–13036
Srivastava AK, Misra N (2014) Unusual properties of novel Li3f3 ring: (Lif2-Li2f) superatomic cluster or lithium fluoride trimer, (Lif)(3)? RSC Adv 4:41260–41265
Donoso R, Cardenas C, Fuentealba P (2014) Ab Lnitio molecular dynamics study of small alkali metal clusters. J Phys Chem A 118:1077–1083
Muz I, Atis M, Canko O (2014) Stochastic search, fragmentation, electronic and reactivity properties of neutral and cationic hydrogenated Li-6 clusters. J Mol Struct 1065:65–73
Ruan W, Xie AD, Wu DL, Luo WL, Yu XG (2014) The geometry structures and electronic properties of Limbn (M+N=12) Clusters. Chinese Phys B 23
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular-orbital methods.25. Supplementary functions for gaussian-basis sets. J Chem Phys 80:3265–3269
Andrae D, Haussermann U, Dolg M, Stoll H, Preuss H (1990) Energy-adjusted abinitio pseudopotentials for the 2nd and 3rd Row transition-elements. Theor Chim Acta 77:123–141
Wang YC, Lv JA, Zhu L, Ma YM (2010) Crystal structure prediction via particle-swarm optimization. Phys Rev B 82
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ã, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) GAUSSIAN 09, Revision B.01. Gaussian Inc., Wallingford
Reed AE, Weinhold F (1983) Natural bond orbital analysis of near-hartree-fock water dimer. J Chem Phys 78:4066–4073
Lu T, Chen FW (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Nyulaszi L (2001) Aromaticity of phosphorus heterocycles. Chem Rev 101:1229–1246
Nyulaszi L, Keglevich G (1994) Study on the aromaticity and reactivity of chlorophosphinines. Heteroat Chem 5:131–137
Nyulaszi L, Veszpremi T, Reffy J, Burkhardt B, Regitz M (1992) Electronic-structure and aromaticity of azaphospholes. J Am Chem Soc 114:9080–9084
Galeev TR, Boldyrev AI (2011) Planarity takes over in the Cxhxp6-X (X=0–6) Series at X=4. Phys Chem Chem Phys 13:20549–20556
Kratschmer W, Lamb LD, Fostiropoulos K, Huffman DR (1990) Solid C-60—a new form of carbon. Nature 347:354–358
Mulliken RS (1962) Criteria for construction of good self-consistent-field molecular orbital wave functions, and significance of Lcao-Mo population analysis. J Chem Phys 36:3428
Politzer P, Mulliken RS (1971) Comparison of 2 atomic charge definitions, as applied to hydrogen fluoride molecule. J Chem Phys 55:5135
Reed AE, Weinstock RB, Weinhold F (1985) Natural-population analysis. J Chem Phys 83:735–746
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge-densities. Theor Chim Acta 44:129–138
Srivastava AK, Misra N (2014) Structures, stabilities, electronic and magnetic properties of small rhxmny (X+Y =2–4) clusters. Comput Theor Chem 1047:1–5
Zubarev DY, Boldyrev AI (2008) Developing paradigms of chemical bonding: adaptive natural density partitioning. Phys Chem Chem Phys 10:5207–5217
Acknowledgments
This work was supported financially by National Natural Science Foundation of China (NSFC Grant No.11204185 and 11334003). The author also acknowledges the National Supercomputing Center in Shenzhen for providing computational resources.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 3224 kb)
Rights and permissions
About this article
Cite this article
Song, Z. First-principle investigation on growth patterns and properties of cobalt-doped lithium nanoclusters. J Mol Model 22, 133 (2016). https://doi.org/10.1007/s00894-016-3002-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3002-4