Abstract
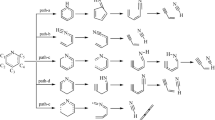
The primary pyrolysis mechanisms of the sodium carboxylate group in sodium benzoate—used as a model compound of brown coal—were studied by performing quantum chemical computations using B3LYP and the CBS method. Various possible reaction pathways involving reactions such as unimolecular and bimolecular decarboxylation and decarbonylation, crosslinking, and radical attack in the brown coal matrix were explored. Without the participation of reactive radicals, unimolecular decarboxylation to release CO2 was calculated to be the most energetically favorable primary reaction pathway at the B3LYP/6-311+G (d, p) level of theory, and was also found to be more energetically favorable than decarboxylation of an carboxylic acid group. When CBS-QBS results were included, crosslinking between the sodium carboxylate group and the carboxylic acid and the decarboxylation of the sodium carboxylate group (catalyzed by the phenolic hydroxyl group) were found to be possible; this pathway competes with unimolecular decarboxylation of the sodium carboxylate group. Provided that H and CH3 radicals are present in the brown coal matrix and can access the sodium carboxylate group, accelerated pyrolysis of the sodium carboxylate group becomes feasible, leading to the release of an Na atom or an NaCO2 radical at the B3LYP/6-311+G (d, p) or CBS-QB3 level of theory, respectively.










Similar content being viewed by others
References
Morgan TJ, Kandiyoti R (2014) Chem Rev 114:1547–1607
Solomon PR, Serio MA, Suuberg EM (1992) Prog Energy Combust Sci 18:133–220
Schafer HNS (1970) Fuel 49:197–213
Quyn DM, Wu H, Bhattacharya SP, Li C-Z (2002) Fuel 81:151–158
Takematsu T, Maude C (1991) Coal gasification for IGCC power generation. Gemini House, London
Eskay TP, Britt PF, Buchanan AC (1996) Energy Fuel 10:1257–1261
Wornat MJ, Sakurovs R (1996) Fuel 75:867–871
Murray JB (1973) Fuel 52:105–111
Schafer HNS (1979) Fuel 58:667–672
Li CZ, Sathe C, Kershaw JR, Pang Y (2000) Fuel 79:427–438
Quyn DM, Wu H, Hayashi J-i, Li C-Z (2003) Fuel 82:587–593
van Eyk PJ, Ashman PJ, Nathan GJ (2011) Combust Flame 158:2512–2523
Sathe C, Pang Y, Li C-Z (1999) Energy Fuel 13:748–755
Wu H, Quyn DM, Li C-Z (2002) Fuel 81:1033–1039
Li CZ (2007) Fuel 86:1664–1683
Domazetis G, Raoarun M, James BD (2006) Energy Fuel 20:1997–2007
Yan G, Zhang Z, Yan K (2013) Mol Phys 111:147–156
Mathews JP, van Duin ACT, Chaffee AL (2011) Fuel Process Technol 92:718–728
Liu S, Zhang Z, Wang H (2012) J Mol Model 18:359–365
Hatcher PG, Breger IA, Szeverenyi N, Maciel GE (1982) Org Geochem 4:9–18
Kim K, Jordan KD (1994) J Phys Chem 98:10089–10094
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) J Phys Chem 98:11623–11627
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) J Comput Chem 17:49–56
Peng C, Schlegel HB (1993) Israel J Chem 33:449–454
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523–5527
Petersson GA, Bennett A, Tensfeldt TG, Al‐Laham MA, Shirley WA, Mantzaris J (1988) J Chem Phys 89:2193–2218
Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA (1999) J Chem Phys 110:2822–2827
Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, revision A.1. Gaussian Inc., Pittsburgh
Ibarra J, Moliner R, Gavilan MP (1991) Fuel 70:408–413
Joseph JT, Forrai TR (1992) Fuel 71:75–80
Blanksby SJ, Ellison GB (2003) Acc Chem Res 36:255–263
Ervin KM, DeTuri VF (2002) J Phys Chem A 106:9947–9956
Czechowski F, Jezierski A (1997) Energy Fuel 11:951–964
Smith GV, Wiltowski T, Phillips JB (1989) Energy Fuel 3:536–537
He YZ, Mallard WG, Tsang W (1988) J Phys Chem 92:2196–2201
Zhang S, Hayashi J-i, Li C-Z (2011) Fuel 90:1655–1661
Acknowledgments
Financial support from the National Natural Science Foundation of China (no. 51404162) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(RAR 612 kb)
Rights and permissions
About this article
Cite this article
Li, J., Zhang, B., Zhang, Z. et al. Quantum chemical investigation of the primary thermal pyrolysis reactions of the sodium carboxylate group in a brown coal model. J Mol Model 20, 2523 (2014). https://doi.org/10.1007/s00894-014-2523-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2523-y