Abstract
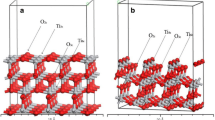
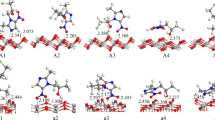
A density functional theory (DFT) method (periodic DMol3) with full geometry optimization was used to study the adsorption of tautomeric forms of tetrazole on anatase TiO2 (101), (100), and (001) surfaces. It was found that the adsorption of tetrazole on the TiO2 surfaces does not proceed via a dissociative process, and negative shifts in the Fermi level of TiO2 were noted upon N-containing heterocycle adsorption. The configuration of the tetrazole during adsorption and the corresponding adsorption energies on different surfaces and sites were estimated. In addition, it was found that tetrazole may be adsorbed on TiO2 surfaces through an interaction between a cation on the surface and a lone pair on the N1 or N2 atom of the tetrazole molecule. The results indicate that the adsorption of tetrazole through the N2 position (leading to the 1H tautomer) on an anatase TiO2 surface is favored over adsorption through the N1 position. In addition, it was observed that the photocatalytic activity of tetrazole-doped TiO2 is higher than that of a pure anatase TiO2 surface.








Similar content being viewed by others
References
Diebold U (2003) Surf Sci Rep 48:53–229
Ranade MR, Navrotski A, Zhang HZ, Banfield JF, Elder SH, Zaban A, Borse PH, Kulkarni SK, Doran GS, Whitfield HJ (2002) Proc Natl Acad Sci USA 99:6476–6481
Hagfelt A, Grätzel M (1995) Chem Rev 95:49–68
Hadjivanov KI, Klissurski DG (1996) Chem Soc Rev 25:61–69
Shklover V, Nazeeruddin MK, Zakeeruddin SM, Barbe C, Kay A, Haibach T, Steurer W, Hermann R, Nissen HU, Gratzel M (1997) Chem Mater 9:430–439
Vittadini A, Selloni A, Rotzinger FP, Gratzel M (1998) Phys Rev Lett 81:2954–2957
Fillinger A, Soltz D, Parkinson BA (2002) J Electrochem Soc 149:146–1156
Gong XQ, Selloni A, Vittadini A (2006) J Phys Chem B 110:2804–2811
Lazzeri M, Vittadini A, Selloni A (2001) Phys Rev B 63:155409–155417
Ushiroda S, Ruzycki N, Lu Y, Spitler MT, Parkinson BA (2005) J Am Chem Soc 127:5158–5168
Wang H, Wu Y, Xu BQ (2005) Appl Catal B Environ 59:139–146
Breysse M, Portefaix JL, Vrinat M (1991) Catal Today 10:489–505
Nakamura I, Negishi N, Kutsuna S, Ihara T, Sugihara S, Takeuchi K (2000) J Mol Catal A 161:205–212
Irie H, Watanabe Y, Hashimoto K (2003) J Phys Chem B 107:5483–5486
Borgarello E, Kiwi J, Gratzel M, Pelizzetti E, Visca M (1982) J Am Chem Soc 104:2996–3002
Yamashita H, Ichihashi Y, Takeuchi M, Kishiguchi S, Anpo M (1999) J Synchrotron Radiat 6:455–457
Gratzel M (1991) Comments Inorg Chem 12:93–111
Pechy P, Rotzinger F, Nazeeruddin MK, Kohle O, Zakeeruddin SM, Humphry-Baker R, Gratzel M (1995) Chem Commun 65–66
Boschloo G, Fitzmaurice D (1999) J Phys Chem 103:2228–2231
Gratzel M (2004) J Photochem Photobiol A Chem 164:3–14
Barbe CJ, Arendse F, Comte P, Jirousek M, Lenzmann F, Shklover V, Gratzel M (1997) J Am Ceram Soc 80:3157–3171
Nakada S, Matsuda M, Kambe S, Saito Y, Kitamura T, Sakata T, Wada Y, Mori H, Yanagida S (2002) J Phys Chem B 106:10004–10010
Kim H, Auyeung RCY, Ollinger M, Kushto GP, Kafafi ZH, Pique A (2006) Appl Phys A 83:73–76
Lavrencic Stangar U, Orel B, Neumann B (2003) J Sol–Gel Sci Technol 26:1113–1118
Onal I, Soyer S, Senkan S (2006) Surf Sci 600:2457–2469
Mguig B, Calatayud M, Minot C (2004) J Mol Struct 709:73–78
Wahab HS, Bredow T, Aliwi SM (2008) J Mol Struct 868:101–108
Wahab HS, Bredow T, Aliwi SM (2008) Chem Phys 354:50–57
Wanbayor R, Deák P, Frauenheim T, Ruangpornvisuti V (2012) Comput Mater Sci 58:24–30
Barreto WJ, Ando RA, Estevão BM, da Silva Zanoni KP (2012) Spectrochim Acta Pt A 92:16–20
Guo J, Watanabe S, Janik MJ, Ma X, Song C (2010) Catal Today 149:218–223
Nilsing M, Lunell S, Persson P, Ojamae L (2005) Surf Sci 582:49–60
Liu H, Zhao M, Lei Y, Pan C, Xiao W (2012) Comput Mater Sci 51:389–395
Ostrovskii V, Koldobskii G, Katritzky AR, Ramsden CA, Scriven EF, Taylor RJ (eds)(2008) Comprehensive heterocyclic chemistry III. Elsevier, Oxford, pp 257–423
Hashimoto Y, Ohashi R, Kurosawa Y, Minami K, Kaji H, Hayashida K, Narita H, Murata S (1998) J Cardiovasc Pharmacol 31:568–575
DeSarro A, Ammendola D, Zappala M, Grasso S, DeSarro G (1995) Antimicrob Agents Chemother 39:232–237
Herr RJ (2002) Bioorg Med Chem 10:3379–3393
Myznikov L, Hrabalek A, Koldobskii G (2007) Chem Heterocycl Compd 43:1–9
Ek F, Wistrand LG, Frejd T (2003) Tetrahedron 59:6759–6769
Flippin LA (1991) Tetrahedron Lett 32:6857–6860
Rhonnstad P, Wensbo D (2002) Tetrahedron Lett 43:3137–3139
Jursic BS, Le Blanc BW (1998) J Heterocycl Chem 35:405–408
John EO, Kirchmeier RL, Shreeve JM (1989) Inorg Chem 28:4629–4633
Zhao-Xu C, Heming X (2000) Int J Quantum Chem 79:350–357
Modarresi-Alam AR, Khamooshi F, Rostamizadeh M, Keykha H, Nasrollahzadeh M, Bijanzadeh HR, Kleinpeter E (2007) J Mol Struct 841:61–66
Dabbagh HA, Chermahini AN (2012) J Iran Chem Soc 9:339–348
Chermahini AN, Nasr-Esfahani M, Dalirnasab Z, Dabbagh HA, Teimouri A (2007) J Mol Struct THEOCHEM 820:7–11
Chermahini AN, Dabbagh HA, Teimouri A (2007) J Mol Struct THEOCHEM 822:33–37
Chermahini AN, Teimouri A, Moaddeli A (2011) Heteroatom Chem 22:168
Chermahini AN, Teimouri A, Salimi Beni A (2011) Struct Chem 22:175–181
Delley B (1990) J Chem Phys 92:508–517
Delley B (1996) J Phys Chem 100:6107–6110
Delley B (2000) J Chem Phys 113:7756–7764
Kim E, Weck PF, Berber S, Tomanek D (2008) Phys Rev B 78:113404–113408
Benedek NA, Snook IK, Latham K, Yarovsky I (2005) J Chem Phys 122:144102–144109
Inada Y, Orita HJ (2008) Comput Chem 29:225–232
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Phys Rev B 46:6671–6687
Cromer DT, Herrington K (1955) J Am Chem Soc 77:4708
Acknowledgments
This work was carried out with financial support from Yasouj University and Isfahan University of Technology.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chermahini, A.N., Hosseinzadeh, B., Beni, A.S. et al. A periodic density functional theory study of tetrazole adsorption on anatase surfaces: potential application of tetrazole rings in dye-sensitized solar cells. J Mol Model 20, 2086 (2014). https://doi.org/10.1007/s00894-014-2086-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2086-y