Abstract
The complexation behavior of nine polyether type podands with a varying number of oxygen donor atoms (4–10) towards the alkali metal cations Li+, Na+ and K+ was studied by quantum chemical methods at the DFT-B3LYP level of theory using the all-electron split-valence 6-311++G(d,p) basis set. The optimized structures of the complexes show a regular increase in the mean cation–oxygen distance with the coordination number. OC–CO dihedral angles of the podand arms were also found to increase with the coordination number and with the size of the cation. Maximum values for the number of strong cation–oxygen interactions (effective coordination numbers) were found for each cation (six for Li+, seven for Na+ and eight for K+). The calculated values for thermodynamic parameters relative to the binding of free and solvated cations to the podands allowed the assessment of binding constants in vacuum, in water and in dichloromethane. The estimated cation extraction constants mimic the experimental extraction trends, but their values are much larger than experimental values. Scale factors were determined to correct the values effectively. For each podand the ratios between the calculated extraction constants of Li+ (or Na+) and the corresponding ones for K+ (seen as extraction selectivities) compare acceptably with the corresponding experimental values.

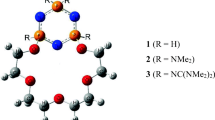
Stereoscopic view of representative structures of a podand and the corresponding K+ complex, highlighting the C-O-C dipole orientations











Similar content being viewed by others
Notes
The symbology used in the present work to designate the podands is based on the number of ethylene glycol (eg) units present in each of the two arms, always bonded to the aromatic ring at ortho positions. So, podand b33 has two equal sized arms consisting of chains with three eg units, comprising a total of eight potentially donor oxygen atoms, bonded at ortho positions to the aromatic ring, and podand b06 has one arm consisting of a chain with six eg units and one methoxy group, comprising a total of eight potentially donor oxygen atoms, bonded at ortho positions to the aromatic ring. Thus, to find the number of potentially donor oxygen atoms of podand b”mn” one just has to perform the sum: m + n + 2.
References
Pedersen CJ (1967) Cyclic polyethers and their complexes with metal salts. J Am Chem Soc 89:7017–7036. doi:10.1021/ja01002a035
Pedersen CJ (1988) The discovery of crown ethers (Nobel lecture). Angew Chem Int Ed Engl 27:1021–1027. doi:10.1002/anie.198810211
Cram D (1988) The design of molecular hosts, guests, and their complexes (Nobel lecture). Angew Chem Int Ed Engl 27:1009–1020. doi:10.1002/anie.198810093
Lehn JM (1988) Supramolecular chemistry—scope and perspectives: molecules, supermolecules, molecular devices (Nobel lecture). Angew Chem Int Ed Engl 27:89–112. doi:10.1002/anie.198800891
Bovill MJ, Chadwick DJ, Sutherland IO (1980) Molecular mechanics calculations for ethers. The conformations of some crown ethers and the structure of the complex of 18-crown-6 with benzylammonium thiocyanate. J Chem Soc Perkin Trans 2 1529–1543. doi:10.1039/P29800001529
Wipff G, Weiner P, Kollman PA (1982) A molecular-mechanics study of 18-crown-6 and its alkali complexes: an analysis of structural flexibility, ligand specificity, and the macrocyclic effect. J Am Chem Soc 104:3249–3258. doi:10.1021/ja00376a001
Grootenhuis PDJ, Kollman PA (1989) Molecular mechanics and dynamics studies of crown ether - cation interactions: free energy calculations on the cation selectivity of dibenzo-18-crown-6 and dibenzo-30-crown-10. J Am Chem Soc 111:2152–2158. doi:10.1021/ja00188a032
Mazor MH, McCammon JA, Lybrand TP (1990) Molecular recognition in nonaqueous solvent. 2. Structural and thermodynamic analysis of cationic selectivity of 18-crown-6 in methanol. J Am Chem Soc 112:4411–4419. doi:10.1021/ja00167a044
Wang J, Kollman PA (1998) Alkali Cation Extraction by 18-Crown-6 and Its Derivatives: A Free Energy Perturbation Study. J Am Chem Soc 120:11106–11114. doi:10.1021/ja980464l
Vayssière P, Wipff G (2003) Importance of counter-ions in alkali and alkaline-earth cation extraction by 18-crown-6: molecular dynamics studies at the water/sc-CO2 interface. Phys Chem Chem Phys 5:2842–2850. doi:10.1039/B303058J
Troxler L, Wipff G (1994) Conformation and dynamics of 18-crown-6, cryptand 222, and their cation complexes in acetonitrile studied by molecular dynamics simulations. J Am Chem Soc 116:1468–1480. doi:10.1021/ja00083a036
Kollman PA, Wipff G, Singh UC (1985) Molecular mechanical studies of inclusion of alkali cations into anisole spherands. J Am Chem Soc 107:2212–2219. doi:10.1021/ja00294a002
Grootenhuis PDJ, Kollmann PA, Groenen LC, Reinhoudt DN, van Hummel GJ, Ugozzoli F, Andreetti GD (1990) Computational study of the structural, energetic, and acid-base properties of calix[4]arenes. J Am Chem Soc 112:4165–4176. doi:10.1021/ja00167a010
Miyamoto S, Kollman PA (1992) Molecular dynamics studies of calixspherand complexes with alkali metal cations: calculation of the absolute and relative free energies of binding of cations to a calixspherand. J Am Chem Soc 114:3668–3674. doi:10.1021/ja00036a015
Varnek A, Wipff GJ (1996) Theoretical calculations of extraction selectivity: alkali cation complexes of calix[4]-bis-crown6 in pure water, chloroform, and at a water/chloroform interface. J Comput Chem 17:1520–1513. doi:10.1002/(SICI)1096-987X(199610
Thuéry P, Nierlich M, Lamare V, Dozol J-F, Asfari Z, Vicens (1997) Potassium and sodium complexes of 1,3-calix[4]-bis-crown-6: crystal and molecular structures, 1H-NMR investigation and molecular dynamics simulation. J Supramol Chem 8:319–332. doi:10.1080/10610279708034951
Sieffert N, Wipff G (2006) Comparing an ionic liquid to a molecular solvent in the cesium cation extraction by a calixarene: a molecular dynamics study of the aqueous interfaces. J Phys Chem B 110:19497–19506. doi:10.1021/jp063045g
Auffinger P, Wipff G (1991) Hydration of the 222 cryptand and 222 cryptates studied by molecular dynamics simulations. J Am Chem Soc 113:5976–5988. doi:10.1021/ja00016a009
Auffinger P, Wipff G (1991) Molecular dynamics simulations on the protonated 222. H+ and 222.2H+ cryptands in water: Endo versus exo conformations. J Incl Phen Macrocyclic Chem 11:71-88. doi:10.1007/BF01073686
Jost P, Galand N, Schurhammer R, Wipff G (2002) The 222 cryptand and its cryptates at the water/“oil” interface: molecular dynamics investigations of concentrated solutions. Phys Chem Chem Phys 4:335–344. doi:10.1039/B104662B
Nazarenko AY, Baulin VE, Lamb JD, Volkova TA, Varnek AA, Wipff G (1999) Solvent extraction of metal picrates by phosphoryl-containing podands. Solvent Extr Ion Exch 17:495–523. doi:10.1080/07366299908934625
Solovév VP, Baulin VE, Strakhova NN, Kazachenko VP, Belski VK, Varnek AA, Volkova TA, Wipff G (1998) Complexation of phosphoryl-containing mono-, bi- and tri-podands with alkali cations in acetonitrile. Structure of the complexes and binding selectivity. J Chem Soc Perkin Trans 2 6:1489–1498. doi:10.1039/A708245B
Leska B, Pankiewicz R, Kira J, Schroeder G (2009) Potentiometric and AM1d studies of a new class of Tri-podands–silver(I) complexes. Supramol Chem 21:218–222. doi:10.1080/10610270802527028
Leska B, Pankiewicz R, Gierczyk B, Schroeder G (2008) Synthesis, structure and application of a new class of Tr-podands derived in phase-transfer catalysis. J Mol Catal A Chem 287:165–170. doi:10.1016/j.molcata.2008.03.011
Leska B, Pankiewicz R, Schroeder G, Gierczyk B, Maciejewski H, Marciniec M (2008) New type of repeated Si-C-podand catalysts for solid-liquid phase transfer reactions. Catal Commun 9:821–825. doi:10.1016/j.catcom.2007.09.012
Leska B, Pankiewicz R, Schroeder G, Maia A (2006) A new type of B-podand catalysts for solid-liquid phase transfer reactions. Tetrahedron Lett 47:5673–5676. doi:10.1016/j.tetlet.2006.06.016
Maia A, Landini D, Betti C, Leska B, Schroeder G (2005) Catalytic activity and anion activation in SN2 reactions promoted by complexes of silicon polypodands. Comparison with traditional polyethers. New J Chem 29:1195–1198. doi:10.1039/B504980F
Maia A, Landini D, Leska B, Schroeder G (2004) Silicon podands: a new class of efficient solid-liquid phase-transfer catalysts. Tetrahedron 60:10111–10115. doi:10.1016/j.tet.2004.06.049
Maia A, Landini D, Leska B, Schroeder G (2003) Silicon podands: powerful metal cation complexing agents and solid-liquid phase-transfer catalysts of new generation. Tetrahedron Lett 44:4149–4151. doi:10.1016/S0040-4039(03)00838-4
Turanov AN, Karandashev VK, Baulin VE, Yarkevich AN, Safronova ZV (2009) Extraction of lanthanides(III) from aqueous nitrate media with tetra-(p-tolyl)[(o-phenylene)oxymethylene]diphosphine dioxide. Solvent Extr Ion Exch 27:551–578. doi:10.1080/07366290903044683
Turanov AN, Karandashev VK, Baulin VE (2008) Effect of anions on the extraction of lanthanides(III) by N, N-dimethyl-N, N-diphenyl-3-hexapentanediamide. Solvent Extr Ion Exch 26:77–99. doi:10.1080/07366290801904871
Turanov AN, Karandashev VK, Baulin VE (2007) Extraction of lanthanides(III) from nitric acid solutions by selected polyfuctional monoacidic organophosphorus compounds. Solvent Extr Ion Exch 25:165–186. doi:10.1080/07366290601169410
Turanov AN, Karandashev VK, Baulin VE (2006) Extraction of rare-earth nitrates by phosphoryl podands. Russ J Inorg Chem 51:1829–1835. doi:10.1134/S0036023606110210
Nazarenko AY, Baulin VE, Lamb JD, Volkova TA, Varnek AA, Wipff G (1999) Solvent extraction of metal picrates by phosphoryl containing podands. Solvent Extr Ion Ex 17:495–523. doi:10.1080/07366299908934625
Turanov AN, Karandashev VK, Baulin VE (1999) Extraction of metal species from nitric acid solutions by phosphoryl-containing podands. Solvenet Extr Ion Exch 17:165–186. doi:10.1080/07366290601169410
Turanov AN, Karandashev VK, Baulin VE (1999) Extraction of rare-earth elements from nitric solutions by phosphoryl-containing podands. Solvent Extr Ion Exch 17:1423–1444. doi:10.1080/07366299908934656
Varnek AA, Ten-Elshof JE, Glebov AS, Solov’ev VP, Baulin VE, Tsvetkov EN (1992) Complexation of lithium and sodium cations with b-phosphorylate ethers, modeling terminal groups of organophosphorus podands: an experimental and theoretical study. J Mol Struct 271:311–325. doi:10.1016/0022-2860(92)80136-6
Yamabe T, Hori K, Akagi K, Fukui K (1979) Stability of crown ether complexes: a mo theoretical study. Tetrahedron 35:1065–1072. doi:10.1016/S0040-4020(01)93724-X
Hori K, Yamada H, Yamabe T (1983) Theoretical study on the nature of the interaction between crown ethers and alkali cations: Relation of interaction energy and ion selectivity. Tetrahedron 39:67–73. doi:10.1016/S0040-4020(01)97631-8
Ha YL, Chakraborty AK (1992) Nature of the interactions of 18-crown-6 with ammonium cations: a computational study. J Phys Chem 96:6410–6419. doi:10.1021/j100194a057
Glendening ED, Feller D, Thompson MA (1994) An ab initio investigation of the structure and alkali metal cation selectivity of 18-crown-6. J Am Chem Soc 116:10657–10669. doi:10.1021/ja00102a035
Feller D (1997) Ab initio study of M+:18-crown-6 microsolvation. J Phys Chem A 101:2723–2731. doi:10.1021/jp9700185
Feller D, Thompson MA, Kendall RA (1997) A theoretical case study of substituent effects and microsolvation on the binding specificity of crown ethers. J Phys Chem A 101:7292–7298. doi:10.1021/jp971509s
Hill SE, Feller D, Glendening ED (1998) Theoretical study of cation/ether complexes: alkali metal cations with 1,2-dimethoxyethane and 12-crown-4. J Phys Chem A 102:3813–3819. doi:10.1021/jp980522p
Ali SKM, Maity DK, De S, Shenoi MRK (2008) Ligands for selective metal ion extraction: a molecular modeling approach. Desalination 232:181–190. doi:10.1016/j.desal.2007.09.017
Diao K-S, Wang H-J, Qiu Z-M (2009) A DFT study on the selective extraction of metallic ions by 12-crown-4. J Solution Chem 38:713–724. doi:10.1007/s10953-009-9406-3
Hou H, Zeng X, Liu X (2009) DFT study of a series of crown-4 ethers and their selectivity trend for alkali metal cations: Li+ and Na+. J Mol Model 15:105–111. doi:10.1007/s00894-008-0379-8
De S, Boda A, Ali SM (2010) Preferential interaction of charged alkali metal ions (guest) within a narrow cavity of cyclic crown ethers (neutral host): a quantum chemical investigation. J Mol Struct THEOCHEM 941:90–101. doi:10.1016/j.theochem.2009.11.009
Becke AD (1993) Density–functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648. doi:10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision C.02. Gaussian Inc, Wallingford CT
Ochterski JW (2000) Thermochemistry in Gaussian. http://www.gaussian.com/g_whitepap/thermo.htm. Accessed 26 November 2010
Glendening ED, Feller D (1995) Cation-water interactions: the M + (H2O)n clusters for alkali metals, M = Li, Na, K, Rb, and Cs J Phys Chem 99:3060–3067. doi:10.1021/j100010a015
Mitani M, Yoshioka Y (2009) A B3LYP study on counterpoise-corrected geometry optimizations for hydrated complexes of [K(H2O) n ]+ and [Na(H2O) n ]+. J Mol Struct THEOCHEM 915:160–169. doi:10.1016/j.theochem.2009.08.030
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. doi:10.1080/00268977000101561
Cui C, Cho SJ, Kim KS (1998) Cation affinities of [1(6)]Starand Model. Comparison with 12-crown-4: crucial role of dipolar moiety orientations. J Phys Chem A 102:1119-1123. doi:10.1021/jp972591u
Hay BP, Rustad JR (1994) Structural criteria for the rational design of selective ligands: extension of the MM3 force field to aliphatic ether complexes of the alkali and alkaline earth cations. J Am Chem Soc 116:6316–6326. doi:10.1021/ja00093a035
Valente M, Sousa SF, Magalhães AL, Freire C (2010) A comparative molecular dynamics study on the complexation of alkali metal cations by a poly-ethylene-glycol type podand in water and in dichloromethane. J Mol Struct THEOCHEM 946:77–82. doi:10.1016/j.theochem.2009.10.025
Valente M, Sousa SF, Magalhães AL, Freire C (2010) Crown-ether type podands as alkali metal cation extractants: influence of the number of oxygens in the chain. J Solut Chem 39:1230–1242. doi:10.1007/s10953-010-9579-9
Feller D, Aprà È, Nichols JA, Bernholdt DE (1996) The structure and binding energy of K+–ether complexes: a comparison of MP2, RI–MP2, and density functional methods. J Chem Phys 105:1940–1950. doi:10.1063/1.472082
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1–2
(DOC 33 kb)
Rights and permissions
About this article
Cite this article
Valente, M., Sousa, S.F., Magalhães, A.L. et al. Complexation of alkali metal cations by crown-ether type podands with applications in solvent extraction: insights from quantum chemical calculations. J Mol Model 17, 3275–3288 (2011). https://doi.org/10.1007/s00894-011-1004-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-011-1004-9