Abstract
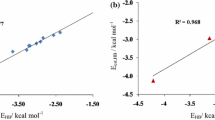
Spin-restricted DFT (X3LYP and B3LYP) and ab initio (MP2(fc) and CCSD(fc)) calculations in conjunction with the Aug-CC-pVDZ and Aug-CC-pVTZ basis sets were performed on a series of hydrogen bonded complexes PN···HX (X = F, Cl, Br) to examine the variations of their equilibrium gas phase structures, energetic stabilities, electronic properties, and vibrational characteristics in their electronic ground states. In all cases the complexes were predicted to be stable with respect to the constituent monomers. The interaction energy (ΔE) calculated using a super-molecular model is found to be in this order: PN···HF > PN···HCl > PN···HBr in the series examined. Analysis of various physically meaningful contributions arising from the Kitaura-Morokuma (KM) and reduced variational space self-consistent-field (RVS-SCF) energy decomposition procedures shows that the electrostatic energy has significant contribution to the over-all interaction energy. Dipole moment enhancement (Δμ) was observed in these complexes expected of predominant dipole-dipole electrostatic interaction and was found to follow the trend PN···HF > PN···HCl > PN···HBr at the CCSD level. However, the DFT (X3LYP and B3LYP) and MP2 levels less accurately determined these values (in this order HF < HCl < HBr). Examination of the harmonic vibrational modes reveals that the PN and HX bands exhibit characteristic blue- and red shifts with concomitant bond contraction and elongation, respectively, on hydrogen bond formation. The topological or critical point (CP) analysis using the static quantum theory of atoms in molecules (QTAIM) of Bader was considered to classify and to gain further insight into the nature of interaction existing in the monomers PN and HX, and between them on H-bond formation. It is found from the analysis of the electron density ρ c , the Laplacian of electron charge density ∇2ρc, and the total energy density (H c ) at the critical points between the interatomic regions that the interaction N···H is indeed electrostatic in origin (ρc > 0, ∇2ρc > 0 and Hc > 0 at the BCP) whilst the bonds in PN (ρc > 0, ∇2ρc > 0 and Hc < 0) and HX ((ρc > 0, ∇2ρc < 0 and Hc < 0)) are predominantly covalent. A natural bond orbital (NBO) analysis of the second order perturbation energy lowering, E(2), caused by charge transfer mechanism shows that the interaction N···H is n(N) → BD*(HX) delocalization.

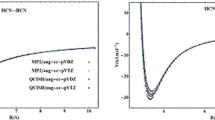
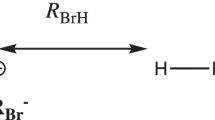
The following figure shows that the N end of molecular phosphorous nitride (PN) is linearly connected (represented by ···) to the H end of molecular hydrogen fluoride (HF) obtained from a RCCSD(fc)/Aug-CC-pVDZ level calculation

Similar content being viewed by others
References
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford Univ Press, New York
Scheiner S (1997) Hydrogen bonding: a theoretical perspective. Oxford University Press, US
Desiraju GR, Sreiner T (2003) The weak hydrogen bond. Oxford Univ Press, New York
Varadwaj PR, Husain MM (2006) Chem Phys Lett 424:227–232
Varadwaj PR (2007) Int J Quantum Chem 107:1194–1204
Rozenberg M, Loewenschuss A, Marcus Y (2000) Phys Chem Chem Phys 2:2699–2702
Ratajczak H (1972) J Phys Chem 76:3991–3992
Kemp DD, Gordon MS (2008) J Phys Chem A 112:4885–4894
Ziurys LM (1987) Astrophys J 321:L81–L85
The encyclopedia of Science. Available via DIALOG. http://www.daviddarling.info/encyclopedia/I/ismols.html
Schnick W, Lücke (1992) Angew Chem Int Ed Engl 31:213–215
Scherer OJ (1992) Angew Chem Int Ed Engl 31:170–171
Fukukawa Y, Mikami O, Okamura M, Hirota Y (1986) Proc Electrochem Soc 86:34 Also see via DIALOG. http://www.patentstorm.us/patents/6586318/description.html
Ahlrichs R, Bär M, Plitt HS, Schnöckel H (1989) Chem Phys Lett 161:179–184
Schnöckel H, Mehner T, Plitt HS, Schunck SC (1989) J Am Chem Soc 111:4578–4582
Atkins RM, Timms PL (1977) Spectrochim Acta Part A 33:853–857
Petrie S (2005) J Phys Chem A 109:6326–6334
Zhengfa HU, Zhenya W, Haiyang LI, Shikang Z (2002) Sci China A 45:1211–1218
Tang SN, Chuang CC, Mollaaghababa R, Klemperer W, Chang HC (1996) J Chem Phys 105:4385–4387
Mckellar ARW, Lu Z (1993) J Mol Spectrosc 161:542–551
Howard NW, Legon AC (1989) J Chem Phys 90:672–678
McMillen C, Bender D, Eliades M, Danzeiers D, Wofford BA, Bevan JB (1988) Chem Phys Lett 152:87–93
Soper PD, Legon AC, Flygare WH (1981) J Chem Phys 74:2138–2142
Legon AC, Soper PD, Keenan MR, Minton TK, Balle TJ, Flygare WH (1980) J Chem Phys 73:583–584
Grabowski SJ (2002) J Mol Struct 615:239–245
McDowell SAC (2006) Chem Phys Lett 424:239–242
Xu X, Goddard WA (2004) Proc Natl Acad Sci 101:2673
Scuseria GE, Schaefer HF III (1989) J Chem Phys 90:3700–3703
Møller C, Plesset MS (1934) Phys Rev 46:618–622
Becke AD (1988) J Chem Phys 88:1053–1062
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Xu X, Goddard WA (2004) J Phys Chem A 108:2305–2313
Xu X, Zhang Q, Muller RP, Goddard WA (2005) J Chem Phys 122:014105
Varadwaj PR, Cukrowki I, Marques HM (2008) J Phys Chem A 112:10657–10666
Varadwaj PR, Cukrowki I, Marques HM (2009) J Mol Struct Theochem 902:21–32
Tsuzuki S, Lüthi HP (2001) J Chem Phys 114:3949–3957
Rao L, Ke H, Xu X, Yan Y (2009) J Chem Theory Comput 5:86–96
Kitaura K, Morokuma K (1976) Int J Quantum Chem 10:325–340
Morokuma K, Kitaura K (1981) In: Politzer P, Truhlar DG (eds) Chemical applications of atomic and molecular electrostatic potentials. Plenum, New York, p 215
Bagus PS, Hermann K, Bauschlicher CW Jr (1984) J Chem Phys 80:4378–4386
Stevens WJ, Fink W (1987) Chem Phys Lett 139:15–22
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Frisch MJ et al (2004) GAUSSIAN 03, Revision C.02. Gaussian Inc, Pittsburg, PA
Schmidt MW, Baldridge KK, Boatz LA, Elbert ST, Gordon MS, Jensen JJ, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
MacMolPlt, version 7.2.1, Bode BM, Gordon MS (1998) J Mol Graphics Mod 16:133–138
Dunning TH Jr (1989) J Chem Phys 90:1007–1023
Kendall RA, Dunning TH Jr, Harrison RJ (1992) J Chem Phys 96:6796–6806
Pople JA (1982) Faraday Discuss Chem Soc 73:7–17
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Blanco F, Alkorta I, Solimannejad M, Elguero J (2009) J Phys Chem A 113:3237–3244
Wong NB, Cheung YS, Wu DY, Ren Y, Tian A, Li WK (2000) J Phys Chem A 104:6077–6082
Varadwaj PR, Cukrowski I, Marques HM (2009) DFT RX3LYP and RPBEPBE studies on the structural, electronic, and vibrational properties of some amino-alcohol ligands. Theochem. doi:10.1016/j.theochem.2009.08.009
Ludwig R (2000) J Mol Liq 84:65–75
Reed AE, Wienhold F, Curtiss LA, Pochatko DJ (1986) J Chem Phys 84:5687–5705
O’Brien SE, Popelier PLA (1999) Can J Chem 77:28–36
Goodman L, Sauers RR (2007) J Comput Chem 28:269–275
Weinhold F (1998) In: Schleyer PVR, Allinger ML, Clark T, Gasteiger J, Kollman PA, Schaefer HF, Schreiner PR (eds) Encyclopedia of computational chemistry, vol 3. John Wiley & Sons, Chichester, p 1792
Glendening EE, Reed AE, Carpenter JE, Weinhold F (2004) NBO 3.0, as implemented in GAUSSIAN 03, Revision C.02. Gaussian Inc, Pittsburg, PA
Keith TA (2008) AIMAll 08.05.04. Available via DIALOG. http://aim.tkgristmill.com
Computational Chemistry Comparison and Benchmark DataBase, NIST Standard Reference Database 101. Available via DIALOG. http://cccbdb.nist.gov/default.htm
Rauhut G, Pulay P (1995) J Am Chem Soc 117:4167–4172
Scott AP, Radom L (1996) J Phys Chem 100:16502–16513
Baker J, Jarzecki AA, Pulay P (1998) J Phys Chem A 102:1412
The Cambridge Structural Database, Radii & Bond lengths. Available via DIALOG. http://www.ccdc.cam.ac.uk/products/csd/radii/table.php4
Bondi A (1964) J Phys Chem 68:441–451
Goubet M, Asselin P, Manceron L, Soulard P, Perchard JP (2003) Phys Chem Chem Phys 5:3591–3594
Andrews L (1984) J Phys Chem 88:2940–2949
McIntosh A, Gallegos AM, Lucchese RR, Bevan JW (1997) J Chem Phys 107:8327–8337
Nesbitt DJ, Lovejoy CM (1992) J Chem Phys 96:5712–5725
Bevan JW, Legon AC, Millen DJ (1980) Proc R Soc Lond A 370:239
Legon AC, Millen DJ, North HM (1987) J Chem Phys 86:2530–2535
Pimentel GC, McClelland AL (1960) The hydrogen bond. Freeman, San Francisco, CA
Murray JS, Ranganathan S, Politzer P (1991) J Org Chem 56:3734–3737
Hagelin H, Murray JS, Brinck T, Berthelot M, Politzer P (1995) Can J Chem 73:483–488
Bader RFW, Essén H (1984) J Chem Phys 80:1943–1960
Bobrov MF, Popova GV, Tsirelson VG (2006) Russ J Phys Chem 80:584–590
Bader RFW (1998) J Phys Chem A 102:7314–7323
Bone RGA, Bader RFW (1996) J Phys Chem 100:10892–10911
Cremer D, Kraka E (1984) Angew Chem Int Ed Engl 23:627–628
Espinosa E, Molins E, Lecomte C (1998) Chem Phys Lett 285:170–173
Jenkins S, Morrison I (2000) Chem Phys Lett 317:97–102
Espinosa E, Alkorta I, Elguero J (2007) Euro J Chem Phys 117:5529
Nakanishi W, Hayashi S, Narahara K (2008) J Phys Chem A 112:13593–13599
Acknowledgments
PRV greatly acknowledges Japan Society for the Promotion of Science (JSPS) for the award of a Postdoctoral Fellowship and funding (Grant No: P 08349). The author thanks Prof. Sean A. C. McDowell and Prof. K. Kawaguchi for helpful discussions and rewarding supports. Thanks are due to the staffs of the Okayama University information science center for providing SX-6i supercomputing facility for GAUSSIAN 03 calculations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varadwaj, P.R. Hydrogen bonding interactions in PN···HX complexes: DFT and ab initio studies of structure, properties and topology. J Mol Model 16, 965–974 (2010). https://doi.org/10.1007/s00894-009-0603-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-009-0603-1