Abstract
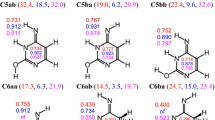
Six uridine and six deoxyuridine isomers were studied at the B3LYP and TD B3LYP theoretical level and 6–31+G(d) basis function. The stability and the excited states of the isomers were studied in order to clarify some known experimental data. It was established that the rotation of the oxo uracil ring in uridine is energetically more likely to occur in the excited state than in the ground state, driven by the bright 1 ππ* state and the dark charge transfer 1nπ* state. Very high energy barriers (on the So) were found for thermal intramolecular proton transfer processes.

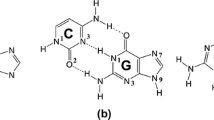
Oxo uracil tautomer of uridine





Similar content being viewed by others
References
Hosein S (2004) Canadian AIDS Treatment Information Exchange (http://www.thebody.com/content/art30244.html, accessed: 14.07.2009)
Walker UA, Langmann P, Miehle N, Zilly M, Klinker H, Petschner F (2004) AIDS 187:1085–1086
Dagan T, Sable C, Bray J, Gerschenson M (2002) Mitochondrion 1:397–412
Carlezon WA Jr, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF (2005) Biol Psychiatry 57:343–350
Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden K (1996) Mol Pharmacol 50:224–229
Qu W, Tabisz GC (2006) J Chem Phys 124:184305–184314
Bureekaew S, Hasegawa J, Nakatsuji H (2006) Chem Phys Lett 425:367–371
Merchan M, Gonzalez-Luque R, Climent T, Serrano-Andres L, Rodriguez E, Reguero M, Pelaez D (2006) J Phys Chem, B 110:26471–26476
Matsika S (2004) J Phys Chem A 108:7584–7590
Santoro F, Barone V, Gustavsson T, Improta R (2006) J Am Chem Soc 128:16312–16322
Lorentzon J, Fülscher MP, Ross BO (1995) J Am Chem Soc 117:9265–9273
Mezzache S, Alves S, Pepe C, Quelquejeu M, Fournier F, Valery JM, Tabet JC (2005) J Mass Spectrom 40:722–730
Green EA, Rosenstein RD, Shiono R, Abraham DJ (1975) Acta Cryst B 31:102–107
Rahman A, Wilson HR (1972) Acta Cryst B 28:2260–2270
Ivanov AY, Krasnokutski SA, Sheina G, Blagoi YP (2003) Spectrochim Acta A 59:1959–1973
Atkins PW (1986) Physical chemistry, 3rd edn. Oxford University Press, Oxford
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, Rev A.7. Gaussian Inc, Pittsburgh PA
Zhurko GA, Zhurko DA (2007) ChemCraft, ver 1.5 (build 282). http://www.softpedia.com/
Clark LB, Peschel GG, Tinoco I Jr (1965) J Phys Chem 69:3615–3618
Okahata Y, Nakayama H (2000) In: Abstacts, 15th Symposium on Biofunctional Chemistry, pp 128–129
Nakayama H, Ohno H, Okahata Y (2001) Chem Commun (Camb) 21:2300–2301
Miles DW, Robins RK, Eyring H (1967) Proc Natl Acad Sci USA 57:1139–1145
Hammond GS (1955) J Am Chem Soc 77:334–338
Leffler JE (1953) Science 117:340–341
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delchev, V.B. Computational (DFT and TD DFT) study of the electron structure of the tautomers/conformers of uridine and deoxyuridine and the processes of intramolecular proton transfers. J Mol Model 16, 749–757 (2010). https://doi.org/10.1007/s00894-009-0593-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-009-0593-z