Abstract
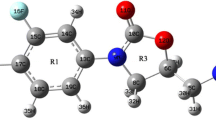
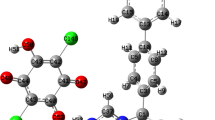
Three of the most frequent antitubercular agents employed against Mycobacterium tuberculosis are: Rifampicin, Isoniazid and Pyrazinamide. It has been proven that the use of these antitubercular agents together, shortens the treatment period from 12–18 months to 6 months [1]. In this work we use a new Density Functional Theory chemistry model called CHIH-DFT (Chihuahua-Heterocycles-Density Functional Theory) that reflects the mixture of Hartree Fock exchange and DFT exchange, according to a mixing parameter based on empirical rules suited for heterocyclic systems. This new chemistry model was used to calculate the molecular structure of these antitubercular compounds, as well as their infrared, UV spectra, chemical reactivity and electronic properties. The UV and infrared spectra were obtained by experimental techniques. The calculated molecular structure, UV and IR spectra values from CHIH-DFT were compared with experimentally obtained values and theoretical studies. These results are in good agreement with experimental and theoretical studies. We also predicted using the relative electrophilicity and relative nucleophilicity concepts as defined by Roy et al. [2] the chemical active sites for the three antitubercular compounds as well as their electronegativity, ionization potential, electron affinity, hardness, dipole moment, EHOMO-ELUMO gap energy, etc.











Similar content being viewed by others
References
Chopra I, Brennan P (1998) Tuberc Lung D 78(2):89–98
Roy RK, Pal S, Hirao K (1999) J Chem Phys 110(17):8236–8245
Dessen A, Quernard A, Blanchard JS, Jacobs Jr WR, Sacchettini JC (1995) Science 267(1995):1638–1641
Manetti F, Corelli F, Biava M, Fioravanti R, Porreta GC, Botta M (2000) Il Fármaco 55:484–491
Secretaria de Salud, Mexico, Boletín epidemiología (2006) http://www.dgepi.salud.gob.mx/boletin/2006/sem02/pdf/cua1y2.pdf
Sociedad Argentina de Pediatria, Criterios de diagnóstico y tratamiento de la tuberculosis infantil (2002) Arch Argent pediatr 100(2):159–178
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision C.02. Gaussian, Wallingford, CT
Foresman JB, Frisch Æ (1996) Exploring chemistry with electronic structure methods, 2nd edn. Gaussian Inc., Pittsburgh, PA
Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA (1999) J Chem Phys 110:2822–2827
Curtiss LA, Raghavachari K, Trucks GW, Pople JA (1991) J Chem Phys 94:7221–7230
Petersson GA, Bennet A, Tensfeldt TG, Al-Laham MA, Shirley WA, Mantzaris J (1988) J Chem Phys 89:2193–2218
Petersson GA, Al-Laham MA (1991) J Chem Phys 94:6081–6090
Adamo C, Barone V (1999) J Chem Phys 110:6158–6170
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Flores-Holguın N, Glossman-Mitnik D (2004) J Mol Struct Theochem 681:77–82
Flores-Holguın N, Glossman-Mitnik D (2005) J Mol Struct Theochem 717:1–3
Flores-Holguın N, Glossman-Mitnik D (2005) J Mol Struct Theochem 723:231–234
Mendoza-Wilson AM, Glossman-Mitnik D (2004) J Mol Struct Theochem 681:71–76
Mendoza-Wilson AM, Glossman-Mitnik D (2005) J Mol Struct Theochem 716:67–72
Rodrıguez-Valdez LM, Martınez-Villafane A, Glossman-Mitnik D (2005) J Mol Struct Theochem 681:83–88
Rodrıguez-Valdez LM, Martınez-Villafane A, Glossman-Mitnik D (2005) J Mol Struct Theochem 716:61–65
Glossman-Mitnik D (2007) Theor Chem Acc 117:57–68
Glossman-Mitnik D (2007) J Mol Model 13:43–46
Lewars E (2003) Computational chemistry - introduction to the theory and applications of molecular and quantum mechanics. Kluwer, Norwell, MA, USA
Stratmann RE, Scuseria GE, Frisch MJ (1998) J Chem Phys 109:8218–8224
Bauernschmitt R, Ahlrichs R (1996) Chem Phys Lett 256:454–464
Casida ME, Jamorski C, Casida KC, Salahub DR (1998) J Chem Phys 108:4439–4449
Thompson MA, Zerner MC (1991) J Am Chem Soc 113:8210–8215
Zerner MC (1991) In: Lipkowitz KB, Boyd DB (eds) Reviews in computational chemistry, vol 2. VCH, New York, pp 313–366
Zerner MC, Correa de Mello P, Hehenberger M (1982) Int J Quant Chem 21:251–258
Hanson LK, Fajer J, Thompson MA, Zerner MC (1987) J Am Chem Soc 109:4728–4730
Anderson WP, Edwards WD, Zerner MC (1986) Inorg Chem 25:2728–2732
Gorelsky SI (2005) SWizard program, http://www.sg-chem.net/
Yang W, Mortier WJ (1986) J Am Chem Soc 108:5708–5711
Hirshfeld FL (1977) Theor Chim Acta 44(2):129–138
Jensen LH (1954) J Am Chem Soc 76:4663–4667
Silverstein RM, Webster FX (1998) Spectrometric identification of organic compounds, 6th edn. Wiley, New York
Savitskaya AV, Paschenko LA, Dobrotvorsky AE (1989) Farmatsiya (Moscow, Russian federation) 38(5):39–44
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Takaki Y, Sasada Y, Watanabe T (1960) Acta Crystallogr 13:693–702
Chis V, Pirnau A, Jurca T, Vasilescu M, Simon S, Cozar O, David L (2005) Chem Phys 316:153–163
Gadret M, Goursolle M, Leger JM, Colleter JC (1975) Acta Crystallogr B 31(5):1454–1462
Ovcharova G, Dimitrova D, Kuneva K (1982) Farmatsiya (Sofia, Bulgaria) 32(4):49–53
Angeloni I, Marzocchi MP, Smulevich G (1984) J Raman Spectrosc 15(2):90–96
Kolandaivel P, Praveena G, Selvarengan P (2005) J Chem Sci 117(5):591–598
Acknowledgements
D. Glossman-Mitnik is a CONACyT and CIMAV researcher. Alejandra Favila and Marco Gallo gratefully acknowledge doctoral and postdoctoral fellowships from the National Science and Technology Council in Mexico (CONACyT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Favila, A., Gallo, M. & Glossman-Mitnik, D. CHIH-DFT determination of the molecular structure infrared spectra, UV spectra and chemical reactivity of three antitubercular compounds: Rifampicin, Isoniazid and Pyrazinamide. J Mol Model 13, 505–518 (2007). https://doi.org/10.1007/s00894-007-0170-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-007-0170-2