Abstract
The rearrangements for 2-phospha-4-silabicyclo[1.1.0]butane, analogous to the valence isomerization of the hydrocarbons bicyclobutane, 1,3-butadiene, and cyclobutene, were studied at the (U)QCISD(T)/6-311+G**//(U)QCISD/6-31G* level of theory. The monocyclic 1,2-dihydro-1,2-phosphasiletes are shown to be the thermodynamically preferred product, in contrast to the isomerization of the hydrocarbons, which favors the 1,3-butadiene structure. Furthermore, an unprecedented direct isomerization pathway to the 1,2-dihydro-1,2-phosphasiletes was identified. This pathway is competitive with the isomerization via the open-chain butadienes and becomes favorable when electron-donating substituents are present on silicon.

2-Phospha-4-silabicyclo[1.1.0]butane can isomerize directly into the more stable P,Si-cyclobutene via an unprecedented [σ2s+σ2a] process, which becomes favorable over the isomerization via the P,Si-butadiene when electron-donating substituents are present on silicon
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
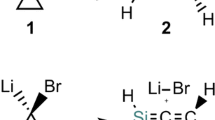
Bicyclo[1.1.0]butane with its strain energy of over 60 kcal mol−1 is a fascinating compound that has attracted the interest of both experimental and theoretical chemists [1]. It is now well established that bicyclo[1.1.0]butane (1) opens to the more stable valence isomer gauche-butadiene (2) by a pericyclic rearrangement, which is characterized by a concerted, asynchronous conrotatory ring opening where the central C–C bond remains intact [2, 3]. This is an allowed [σ2s+σ2a] conrotatory rearrangement according to the Woodward–Hoffmann (W–H) orbital-symmetry rules [4–6], affording kinetic intermediate 2 that can easily rotate to s-trans-1,3-butadiene (3). The activation barrier of 41.5 kcal mol−1 calculated at the multiconfiguration self-consistent field level of theory [2] agrees closely with the experimental value of 40.6 kcal mol−1 [7, 8]. The disrotatory, W–H forbidden, thermal ring opening of 1 is less favorable, and was calculated to be about 15 kcal mol−1 higher in energy [2]. Another rearrangement is also feasible; stretching of the central C–C bond leads to a planar singlet diradical transition structure for inversion, which is also a higher energy process with a barrier of 47.4 kcal mol−1 [9].

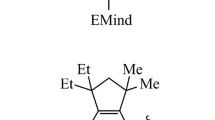
Valence isomer cyclobutene (4) is of intermediate stability between 1 and 3 and converts thermally to gauche-butadiene 2 by an electrocyclic ring opening [10, 11]. This pericyclic rearrangement follows a W–H allowed concerted, conrotatory pathway. The calculated activation barrier at the MP2/6-311G** level of theory of 33.7 kcal mol−1 [12–14] for this process is in agreement with the experimental value of 32.9±0.5 kcal mol−1 [10, 11]. Usually for the ring opening of cyclobutenes, steric effects dominate the preference for inward versus outward rotation [15]. However, electronic effects can also dictate this rearrangement, as was reported very recently for the sterically hindered substrate 5, which prefers to react via the more crowded inward rotatory pathway, leading mainly to butadiene 6 (Scheme 1) [16, 17].
Bicyclo[1.1.0]butanes with main-group hetero-elements in the ring have also received considerable attention [18]. However, little is known about the phosphorus-containing analogues [19–22]. In our ongoing research on small strained organophosphorus ring systems, we became interested in the yet unknown 2-phospha-4-silabicyclo[1.1.0]butanes, whose occurrence we reported as a reactive intermediate recently [23, 24]. Valence isomerization of the 2-phospha-4-silabicyclo[1.1.0]butane 9 to the 1,2-dihydro-1,2-phosphasiletes 10a,b was indicated by reacting 1H-phosphirene 8 with silylene Si[(NCH t2 Bu)2C6H4-1,2] [≡Si(NN)] (Scheme 2).
SCS-MP2/6-311+G** calculations on B3LYP/6-31G* model structures show that the intermediate 2-phospha-4-silabicyclo[1.1.0]butane isomerizes directly, via an unprecedented W–H allowed [σ2s+σ2a] process, to the thermodynamically preferred 1,2-dihydro-1,2-phosphasilete [23, 24]. This pathway is favored over the concerted, asynchronous conrotatory ring opening leading to s-trans-1-phospha-4-sila-1,3-butadiene [25].
Here, we report on the isomerization of 2-phospha-4-silabicyclo[1.1.0]butane A to its valence isomers 1-phospha-4-sila-1,3-butadiene B and 1,2-dihydro-1,2-phosphasilete C (only one other synthesis of 1,2-dihydro-1,2-phosphasiletes was reported: [26–28]), using high-level ab initio calculations at the (U)QCISD(T)/6-311+G**//(U)QCISD/6-31G* level of theory. We will compare the differences between a direct A→C pathway versus the isomerization via butadiene B. In addition, the influence of substituents on silicon on the rearrangements will also be discussed.

Computational details
All calculations were performed using the GAUSSIAN 98 [29] suite of programs. Geometries were optimized using the standard 6-31G* basis set at the (U)MP2 and (U)QCISD [30, 31] level of theory, while single-point calculations were preformed at the (U)QCISD(T)/6-311+G** level using the (U)QCISD/6-31G* geometries. First and second order energy derivatives were computed to confirm that minima or transition structures had been located at the (U)MP2/6-31G* level. Intrinsic reaction coordinate driving calculations were performed at the (U)MP2/6-31G* level to establish the connections between transition structures and minima. The total energies calculated at the (U)MP2, (U)QCISD, and (U)QCISD(T) levels were corrected for the (U)MP2/6-31G* level zero-point energies scaled by a factor of 0.967 [32].
Results and discussion
First, we investigated the rearrangements of bicyclo[1.1.0]butane (1) and cyclobutene (4) into the more stable s-trans-1,3-butadiene (3) at the (U)QCISD(T)/6-311+G**//(U)QCISD/6-31G* level of theory (this method gives similar energies when compared to the CASSCF(10,10)/6-31G* level of theory as was reported for the isomerization of 2-oxabicyclo[1.1.0]butane: [33]), since no complete study of the valence isomerizations of all C4H6 isomers at the same level of theory were reported to date. Subsequently, we investigated the rearrangements of the 2-phospha-4-silabicyclo[1.1.0]butanes, where the effects of heteroatom substitution on the characteristics of the rearrangements become apparent.
Bicyclo[1.1.0]butane (1) leads to gauche-butadiene 2 via a concerted, asynchronous conrotatory ring opening [2, 3], which has a barrier of 39.2 kcal mol−1, and is exothermic by 26.0 kcal mol−1 (Fig. 1). This closed-shell rearrangement is favored over the corresponding diradical open-shell pathway (ΔE ‡=43.2 kcal mol−1, <S 2>=0.85). In addition, cyclobutene (4) also gives 2 via a synchronous (Cs symmetry) conrotatory ring opening [12–14] that requires 32.8 kcal mol−1, and is exothermic by 9.9 kcal mol−1. Both calculated reaction barriers are in excellent agreement with the experimental values of 40.6 kcal mol−1 [7, 8] and 32.9 kcal mol−1 [10, 11], respectively.
Relative QCISD(T)/6-311+G**//QCISD/6-31G* (UQCISD(T)/6-311+G**//UQCISD/6-31G* in parenthesis) energies (ZPE corrected, in kcal mol−1) for the rearrangements of 1 and 4 into 2. Selected bond lengths [Å], angles and torsion angles [°] of 1 (C2v): C1–C2 1.498, C2–C3 1.494, C2–C1–C3 59.8, C1–C2–C3–C4 121.9; 2 (C2): C1–C2 1.342, C2–C3 1.479, C3–C4 1.342, C1–C2–C3–C4 37.9; 4 (C2v): C1–C2 1.520, C1–C4 1.570, C2–C3 1.346, C1–C2–C3 94.2; TS1–2 (closed-shell): C1–C2 1.403, C1–C3 2.344, C2–C3 1.542, C2–C4 1.569, C2–C1–C3 39.4; TS4–2 (C2): C1–C2 1.430, C1–C4 2.150, C2–C3 1.379, C1–C2–C3–C4 21.7
The kinetic gauche-butadiene 2 can easily transform into its enantiomer 2′ via the planar s-cis-1,3-butadiene (TS2-2′) [2, 34] with a barrier of only 0.7 kcal mol−1, or can rotate to the more stable trans-butadiene 3 (ΔE ‡=2.5 kcal mol−1) with an exothermicity of 2.6 kcal mol−1 (Fig. 2) [12–14]. The geometrical parameters of the optimized structures 1, 3, and 4 at the QCISD/6-31G* level of theory are in excellent agreement with the experimental estimates (experimental structures—1, 3, and 4: [35–37]).
Relative QCISD(T)/6-311+G**//QCISD/6-31G* energies (ZPE corrected, in kcal mol−1) for the rearrangements of 2. Selected bond lengths [Å], angles and torsion angles [°] of 3 (C2 h): C1–C2 1.343, C2–C3 1.467, C1–C2–C3 123.8; TS2–3 (C2): C1–C2 1.340, C2–C3 1.490, C1–C2–C3–C4 101.9; TS2–2′ (C2v): C1–C2 1.430, C2–C3 1.379, C1–C2–C3–C4 0.0
Incorporating heteroatoms into the bicyclo[1.1.0]butane framework has a profound impact. We found that 2-phospha-4-silabicyclo[1.1.0]butane (11) opens with a modest exothermicity (0.4 kcal mol−1) directly to valence isomer s-1-phospha-4-sila-1,3-butadiene (12) in its trans configuration via a concerted, asynchronous conrotatory ring opening. In this process, the P–C2 bond becomes elongated well before that of the Si–C1 bond (Fig. 3).
Relative QCISD(T)/6-311+G**//QCISD/6-31G* (UQCISD(T)/6-311+G**//UQCISD/6-31G* in parenthesis) energies (ZPE corrected, in kcal mol−1) for the rearrangements of 11 into 14. Selected bond lengths [Å], angles and torsion angles [°] of 11 (Cs): P1–C1 1.852, Si1–C1 1.840, C1–C2 1.548, C1–P1–C2 49.4, C1–Si1–C2 49.7, P1–C1–C2–Si1 119.0; TS11–12: P1–C1 1.782, P1–C2 2.664, Si1–C1 1.982, Si1–C2 1.785; 12 (Cs): P1–C1 1.708, Si1–C2 1.741, C1–C2 1.443; TS12–13: P1–C1–C2–Si1 103.3; 13: P1–C1–C2–Si1 36.3; TS13–14: P1–C1 1.736, P1–Si1 3.001, Si1–C2 1.774, C1–C2 1.414, P1–C1–C2–Si1 34.1; 14: P1–C1 1.869, P1–Si1 2.290, Si1–C2 1.872, C1–C2 1.354
The activation barrier of 38.8 kcal mol−1 is very similar to the calculated activation barrier of 39.2 kcal mol−1 for the [σ2s+σ2a] process in bicyclo[1.1.0]butane (1). The closed-shell rearrangement 11→12 is favored over the corresponding diradical open-shell pathway (ΔE ‡=41.3 kcal mol−1, <S 2>=0.97).
s-Trans-butadiene 12 can transform into the slightly less stable gauche-butadiene 13 (ΔE=2.6 kcal mol−1) with an energy barrier of 7.5 kcal mol−1. Subsequently, butadiene 13 can isomerize via a conrotatory electrocyclic ring closure to the much more stable 1,2-dihydro-1,2-phosphasilete (14) (ΔE=−23.9 kcal mol−1), with a rearrangement barrier of only 3.2 kcal mol−1. Clearly, if a 1-phospha-4-sila-butadiene is to be formed from 11, it will rearrange to the four-membered ring structure 14. We conclude that in contrast to the hydrocarbons, where butadiene 3 is the favored product, the P,Si-derivatives 12 and 13 are not likely candidates to be observed on rearranging bicyclic compound 11.
As 14 is thermodynamically the preferred valence isomer, we also explored whether it could be formed directly from bicyclic 11. Indeed, forcing an asynchronous conrotatory ring opening with an initial SiH2-group rotation resulted in a transition structure TS11–14 for the direct rearrangement of 11 into 14 (Fig. 4). The barrier of 39.0 kcal mol−1 for this closed-shell process is similar to the conversion via the P,Si-butadienes (ΔE ‡=38.8 kcal mol−1, Fig. 3)Footnote 1. The rearrangement via TS11–14 obeys the orbital symmetry rules and can be described as a [σ2s+σ2a] process. Such a pathway is unprecedented for the isomerization of the carbon analogue bicyclo[1.1.0]butane (1) [2], for which s-trans-1,3-butadiene is the favored product.
Due to the similarities in activation energy for the conversions 11→12 and 11→14 at the QCISD(T)/6-311+G**//QCISD/6-31G* level of theory, we have also incorporated in our computational model the cyclic diamine HN-C=C-NH as substituent on silicon to investigate the effect of donating N atoms, which are also present in our experimental system [23, 24] on the rearrangements.
Substituted 2-phospha-4-silabicyclo[1.1.0]butane 15 leads to its valence isomer s-trans-1-phospha-4-sila-1,3-butadiene 16 via a concerted, asynchronous conrotatory ring opening (ΔE ‡=39.0 kcal mol−1), with a modest endothermicity of 1.5 kcal mol−1 (Fig. 5). The associated transition structure TS15–16 shows features similar to the parent analogue TS11–12, and the closed-shell rearrangement 15→16 is favored over the corresponding diradical open-shell pathway (ΔE ‡=46.1 kcal mol−1, <S 2>=0.97)Footnote 2. In addition, s-trans-butadiene 16 can transform into the slightly more stable planar cis-butadiene 17 (ΔE=−1.5 kcal mol−1), which is now an energy minimum, with an energy barrier of only 8.2 kcal mol−1.
Relative QCISD(T)/6-311+G**//QCISD/6-31G* (UQCISD(T)/6-311+G**//UMP2/6-31G* in parenthesis) energies (ZPE corrected, in kcal mol−1) for the rearrangements of 15 into 18. Selected bond lengths [Å], angles and torsion angles [°] of 15 (Cs): P1–C1 1.852, Si1–C1 1.823, Si1–N1 1.730, C1–C2 1.613, C1–P1–C2 51.6, C1–Si1–C2 52.5, P1–C1–C2–Si1 122.1; TS15–16: P1–C1 1.768, P1–C2 2.590, Si1–C1 1.977, Si1–C2 1.748, Si1–N1 1.726; 16 (Cs): P1–C1 1.718, Si1–C2 1.724, Si1–N1 1.717, C1–C2 1.434; TS16–17: P1–C1–C2–Si1 98.2; 17 (Cs): P1–C1 1.727, Si1–C2 1.732, Si1–N1 1.710, Si1–N2 1.717; TS17–18: P1–C1 1.743, P1–Si1 3.103, Si1–C2 1.765, Si1–N1 1.719, C1–C2 1.406, P1–C1–C2–Si1 24.8; 18: P1–C1 1.867, P1–Si1 2.309, Si1–C2 1.867, Si1–N1 1.741, C1–C2 1.356
Subsequently, 17 can isomerize via a conrotatory electrocyclic ring closure to the much more stable 1,2-dihydro-1,2-phosphasilete 18 (ΔE=−25.6 kcal mol−1) with a minute barrier of only 1.3 kcal mol−1. The geometrical parameters of the optimized 18 are in good agreement with the single-crystal X-ray analysis of 10a [23, 24].
Interestingly, the direct valence isomerization now becomes favorable, and 2-phospha-4-silabicyclo[1.1.0]butane 15 gives cyclobutene derivative 18 (ΔE ‡=27.7 kcal mol−1) via a W–H allowed [σ2s+σ2a] process, with an exothermicity of 25.6 kcal mol−1 (Fig. 6).
The lower barrier for the direct conversion 15→18 compared to that of the parent 11→14 can be ascribed to the presence of the donating amino groups on silicon. Generally, π-donor (e.g., NH2) and σ-acceptor (e.g., F) substituents destabilize three-membered rings, making them more reactive, as indicated by their increased ring strain [38, 39]. This is also evident for the 15→18 conversion by an increased exothermicity (ΔE 11→14 =21.7 kcal mol−1; ΔE 15→18 =25.6 kcal mol−1). Additionally, the analogous rearrangement for the fluoro-substituted 2-phospha-4-silabicyclo[1.1.0]butane 19 confirms this trend (ΔE 19→20 =28.1 kcal mol−1, Fig. 7). Furthermore, the associated transition state of this novel pathway is stabilized by the electron-donating N-heterocyclic substituent on silicon (ΔE ‡ 11→14 =39.0 kcal mol−1; ΔE ‡ 15→18 =27.7 kcal mol−1; ΔE ‡ 19→20 =35.0 kcal mol−1).
Relative QCISD(T)/6-311+G**//QCISD/6-31G* energies (ZPE corrected, in kcal mol−1) for the direct rearrangement of 19 into 20. Selected bond lengths [Å] and torsion angles [°] of 19 (Cs): P1–C1 1.852, Si1–C1 1.797, Si1–F1 1.600, C1–C2 1.631, C1–P1–C2 52.2, C1–Si1–C2 54.0, P1–C1–C2–Si1 122.0; TS19–20: P1–C1 1.834, P1–Si1 2.383, Si1–C2 1.758, Si1–F1 1.613, Si1–F2 1.610, C1–C2 1.457, P1–C1–C2–Si1 77.5; 20: P1–C1 1.879, P1–Si1 2.252, Si1–C2 1.841, Si1–F1 1.607, C1–C2 1.357
Conclusions
Hetero substitution changes the stability of the valence isomers of bicyclo[1.1.0]butane (1). 2-Phospha-4-silabicyclo[1.1.0]butane (11) is the least stable isomer and 1,2-dihydro-1,2-phosphasilete (14) the most stable one at the QCISD(T)/6-311+G**//QCISD/6-31G* level of theory [40]. Two reaction pathways for the thermal isomerization of 2-phospha-4-silabicyclo[1.1.0]butane (11) have been found: (a) a three-step process starting with a barrier of 38.8 kcal mol−1 for the concerted, asynchronous conrotatory ring opening of 11 to s-trans-1-phospha-4-sila-1,3-butadiene (12), followed by a conformational change to the gauche isomer 13 and a subsequent conrotatory electrocyclic ring closure to 14, and (b) a direct transformation of 11 into 14 via a [σ2s+σ2a] process with a barrier of 39.0 kcal mol−1 which becomes favorable when electron-donating substituents are present on silicon. This latter path is unprecedented for the analogous isomerization of bicyclo[1.1.0]butane.
Cartesian coordinates and energies of all stationary points are available in the electronic supplementary material.
Notes
No competitive open-shell rearrangement is present for TS11–14.
Open-shell TS15–16 was calculated at the UQCISD(T)/6-311+G**//UMP2/6-31G* level of theory.
References
Hoz S (1987) Bicyclo[1.1.0]butane. In: Rappoport Z (ed) The chemistry of the cyclopropyl group, Part 2, chapter 19. Wiley, Chichester
Nguyen KA, Gordon MS (1995) J Am Chem Soc 117:3835–3847
Shevlin PB, McKee ML (1988) J Am Chem Soc 110:1666–1671
Woodward RB, Hoffmann R (1969) Angew Chem 81:797–870
Woodward RB, Hoffmann R (1969) Angew Chem Int Ed 8:781–853
Woodward RB, Hoffmann R (1970) The conservation of orbital symmetry. Academic, New York
Frey HM, Stevens IDR (1965) Trans Faraday Soc 61:90–94
Srinivasan R, Levi AA, Haller I (1965) J Phys Chem 69:1775–1777
Nguyen KA, Gordon MS, Boatz JA (1994) J Am Chem Soc 116:9241–9249
Cooper W, Walters WD (1958) J Am Chem Soc 80:4220–4224
Carr RW Jr, Walters WD (1965) J Phys Chem 69:1073–1075
Deng L, Ziegler T (1995) J Phys Chem 99:612–618
Wiest O, Houk KN, Black KA, Thomas BE IV (1995) J Am Chem Soc 117:8594–8599
Spellmeyer DC, Houk KN (1988) J Am Chem Soc 110:3412–3416
Niwayama S, Kallel EA, Spellmeyer DC, Sheu C, Houk KN (1996) J Org Chem 61:2813–2825
Murakami M, Hasegawa M (2004) Angew Chem 116:4981–4984
Murakami M, Hasegawa M (2004) Angew Chem Int Ed 43:4873–4876
Iwamoto T, Yin D, Kabuto C, Kira M (2001) J Am Chem Soc and references therein 123:12730–12731
Tebby JC (2001) Bicyclic and polycyclic systems with a ring junction phosphorus atom. In: Mathey F (ed) Phosphorus-carbon heterocyclic chemistry: the rise of a new domain. Pergamon, Amsterdam, pp 683
Niecke E, Fuchs A, Nieger M (1999) Angew Chem 111:3213–3216
Niecke E, Fuchs A, Nieger M (1999) Angew Chem Int Ed 38:3028–3031
Jones C, Platts JA, Richards AF (2001) Chem Commun 663–664
Slootweg JC, de Kanter FJJ, Schakel M, Ehlers AW, Gehrhus B, Lutz M, Mills AM, Spek AL, Lammertsma K (2004) Angew Chem 116:3556–3559
Slootweg JC, de Kanter FJJ, Schakel M, Ehlers AW, Gehrhus B, Lutz M, Mills AM, Spek AL, Lammertsma K (2004) Angew Chem Int Ed 43:3474–3477
Slootweg JC, Ehlers AW, Lammertsma K (2004) Phosphorus, sulfur and silicon 179:803–807
Haber S, Boese R, Regitz M (1990) Angew Chem 102:1523–1525
Haber S, Boese R, Regitz M (1990) Angew Chem Int Ed 29:1436–1438
Haber S, Schmitz M, Bergsträßer U, Hoffmann J, Regitz M (1999) Chem Eur J 5:1581–1589
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Rega N, Salvador P, Dannenberg JJ, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2002) Gaussian 98 (Revision A.11.4). Gaussian Inc., Pittsburgh
Gauss J, Cremer C (1988) Chem Phys Lett 150:280–286
Lee TJ, Rendell AP, Taylor PR (1990) J Phys Chem 94:5463–5468
Scott AP, Radom L (1996) J Phys Chem 100:16502–16513
Okovytyy S, Gorb L, Leszczynski J (2001) Tetrahedron 57:1509–1513
Breulet J, Lee TJ, Schaefer HF III (1984) J Am Chem Soc 106:6250–6253
Bock CW, Panchenko YN (1989) J Mol Struct 187:69–82
Kuchitsu K, Fukuyama T, Morino Y (1968) J Mol Struct 1:463–479
Bak B, Led JJ, Nygaard L, Rastrup-Andersen J, Sørensen GO (1969) J Mol Struct 3:369–378
Bach RD, Dmitrenko O (2002) J Org Chem 67:2588–2599
Cremer D, Kraka E (1985) J Am Chem Soc 107:3811–3819
Driess M, Pritzkow H, Rell S, Janoschek R (1997) For P2Si2H4 the diphospha-disilabicyclo[1.1.0]butane isomers are the most stable ones. Inorg Chem 36:5212–5217
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Dr. Paul von Ragué Schleyer on the occasion of his 75th birthday.
Electronic supplementary material
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Slootweg, J.C., Ehlers, A.W. & Lammertsma, K. Valence isomerization of 2-phospha-4-silabicyclo[1.1.0]butane: a high-level ab initio study. J Mol Model 12, 531–536 (2006). https://doi.org/10.1007/s00894-005-0041-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-005-0041-7