Abstract
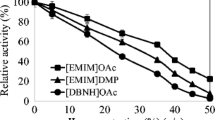
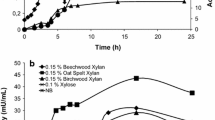
GH10 xylanase from Thermoascus aurantiacus strain SL16W (TasXyn10A) showed high stability and activity up to 70–75 °C. The enzyme’s half-lives were 101 h, 65 h, 63 min and 6 min at 60, 70, 75 and 80 °C, respectively. The melting point (T m), as measured by DSC, was 78.5 °C, which is in line with a strong activity decrease at 75–80 °C. The biomass-dissolving ionic liquid 1-ethyl-3-methylimidazolium acetate ([emim]OAc) in 30 % concentration had a small effect on the stability of TasXyn10A; T m decreased by only 5 °C. It was also observed that [emim]OAc inhibited much less GH10 xylanase (TasXyn10A) than the studied GH11 xylanases. The K m of TasXyn10A increased 3.5-fold in 15 % [emim]OAc with xylan as the substrate, whereas the approximate level of V max was not altered. The inhibition of enzyme activity by [emim]OAc was lesser at higher substrate concentrations. Therefore, high solid concentrations in industrial conditions may mitigate the inhibition of enzyme activity by ionic liquids. Molecular docking experiments indicated that the [emim] cation has major binding sites near the catalytic residues but in lower amounts in GH10 than in GH11 xylanases. Therefore, [emim] cation likely competes with the substrate when binding to the active site. The docking results indicated why the effect is lower in GH10.







Similar content being viewed by others
References
Anbarasan S, Jänis J, Paloheimo M, Laitaoja M, Vuolanto M, Karimäki J, Vainiotalo P, Leisola M, Turunen O (2010) Effect of pH, glycosylation and additional domains on the thermostability of family 10 xylanase of Thermopolyspora flexuosa. Appl Environ Microbiol 76:356–360
Andrade C (1996) Production and characterization of extremely thermostable xylanolytic and amylolytic enzymes from the hyperthermophilic archaeon Pyrodictium abyssi. Ph.D. thesis, Technical University Hamburg-Harburg, Hamburg
Bailey MJ, Bieley P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270
Beg QK, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338
Biely P, Markovic O, Mislovicova C (1985) Sensitive detection of endo-1,4-β-glucanases and endo-1,4-β-xylanases in gels. Anal Chem 144:147–151
Brandt A, Graesvik J, Hallett JP, Welton T (2013) Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem 15:550–583
Brienzo M, Arantes V, Milagres AMF (2008) Enzymology of the thermophilic ascomycetous fungus Thermoascus aurantiacus. Fungal Biol Rev 22:120–130
Cesar T, Mrša V (1996) Purification and properties of the xylanase produced by Thermomyces lanuginosus. Enzyme Microb Technol 19:289–296
Collins T, Gerday C, Feller G (2005) Xylanase, xylanase families and extremophilic xylanases. FEMS Microb Rev 29:3–23
Grosdidier A, Zoete V, Michielin O (2011) SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res 39:W270–W277
Hu J, Arantes V, Saddler J (2011) The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: is it an additive or synergistic effect? Biotechnol Biofuels 4:36
Jacobs A, Dahlman O (2001) Characterization of the molar masses of hemicelluloses from wood and pulps employing size exclusion chromatography and matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Biomacromolecules 2:894–905
Jaeger V, Pfaendtner J (2013) Structure, dynamics and activity of xylanase solvated in binary mixtures of ionic liquid and water. ACS Chem Biol 117:2662–2670
Kongbuntad W, Khanongnuch C, Lumyong S (2006) Efficacy of xylanase supplementation produced from Thermoascus aurantiacus SL16W in diet on Thai native chicken performance. Int J Poult Sci 5:463–469
Kumar V, Satyanarayana T (2013) Biochemical and thermodynamic characteristics of thermo-alkali-stable xylanase from a novel polyextremophilic Bacillus halodurans TSEV1. Extremophiles 17:797–808
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leisola M, Turunen O (2007) Protein engineering: opportunities and challenges. Appl Microbiol Biotechnol 75:1225–1232
Li H, Kankaanpää A, Hummel M, Sixta H, Xiong H, Turunen O (2013) Thermostabilization of extremophilic Dictyoglomus thermophilum GH11 xylanase by an N-terminal disulphide bridge and the effect of ionic liquid [emim]OAc on the enzymatic performance. Enzyme Microb Technol 53:414–419
Lo Leggio L, Kalogiannis S, Eckert K, Teixeira SC, Bhat MK, Andrei C, Pickersgill RW, Larsen S (2001) Substrate specificity and subsite mobility in T. aurantiacus xylanase 10A. FEBS Lett 509:303–308
Mäki-Arvela P, Anugwom I, Virtanen P, Sjöholm R, Mikkola JP (2010) Dissolution of lignocellulosic materials and its constituents using ionic liquids—a review. Ind Crops Prod 32:175–201
McClendon SD, Batth T, Petzold CJ, Adams PD, Simmons BA, Singer SW (2012) Thermoascus aurantiacus is a promising source of enzymes for biomass deconstruction under thermophilic conditions. Biotechnol Biofuels 5:54
Natesh R, Bhanumoorthy P, Vithayathil PJ, Sekar K, Ramakumar S, Viswamitra MA (1999) Crystal structure at 1.8 Å resolution and proposed amino acid sequence of a thermostable xylanase from Thermoascus aurantiacus. J Mol Biol 288:999–1012
Natesh R, Manikandan K, Bhanumoorthy P, Viswamitra MA, Ramakumar S (2003) Thermostable xylanase from Thermoascus aurantiacus at ultrahigh resolution (0.89 Å) at 100 K and atomic resolution (1.11 Å) at 293 K refined anisotropically to small-molecule accuracy. Acta Crystallogr D Biol Crystallogr 59:105–117
Nordwald EM, Kaar JL (2013) Stabilization of enzymes in ionic liquids via modification of enzyme charge. Biotechnol Bioeng 110:2352–2360
Park JI, Steen EJ, Burd H, Evans SS, Redding-Johnson AM, Batth T, Benke PI, D’haeseleer P, Sun N, Sale KL, Keasling JD, Lee TS, Petzold CJ, Mukhopadhyay A, Singer SW, Simmons BA, Gladden JM (2012) A thermophilic ionic liquid-tolerant cellulase cocktail for the production of cellulosic biofuels. PLoS One 7:e37010
Pinkert A, Marsh KN, Pang S, Staiger MP (2009) Ionic liquids and their interaction with cellulose. Chem Rev 109:6712–6728
Quijada-Maldonado E, van der Boogaart S, Lijbers JH, Meindersma GW, de Haan AB (2012) Experimental densities, dynamic viscosities and surface tensions of the ionic liquids series 1-ethyl-3-methylimidazolium acetate and dicyanamide and their binary and ternary mixtures with water and ethanol at T = (298.15–343.15 K). J Chem Thermodyn 51:51–58
Raddadi N, Cherif A, Daffonchio D, Fava F (2013) Halo-alkalitolerant and thermostable cellulases with improved tolerance to ionic liquids and organic solvents from Paenibacillus tarimensis isolated from the Chott El Fejej, Sahara desert, Tunisia. Bioresour Technol 150C:121–128
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
Shi J, Gladden JM, Sathitsuksanoh N, Kambam P, Sandoval L, Mitra D, Zhang S, George A, Singer SW, Simmons BA, Singh S (2013a) One-pot ionic liquid pretreatment and saccharification of switchgrass. Green Chem 15:2579–2589
Shi H, Zhang Y, Li X, Huang Y, Wang L, Wang Y, Ding H, Wang FA (2013b) Novel highly thermostable xylanase stimulated by Ca2+ from Thermotoga thermarum: cloning, expression and characterization. Biotechnol Biofuels 18:26
Teixeira RS, da Silva AS, Kim HW, Ishikawa K, Endo T, Lee SH, Bon EP (2013) Use of cellobiohydrolase-free cellulase blends for the hydrolysis of microcrystalline cellulose and sugarcane bagasse pretreated by either ball milling or ionic liquid [Emim][Ac]. Bioresour Technol 149:551–555
Teugjas H, Väljamäe P (2013) Product inhibition of cellulases studied with 14C-labeled cellulose substrates. Biotechnol Biofuels 6:104
Thomas MF, Li LL, Handley-Pendleton JM, van der Lelie D, Dunn JJ, Wishart JF (2011) Enzyme activity in dialkyl phosphate ionic liquids. Bioresour Technol 102:11200–11203
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31:455–461
Wahlström W, King A, Parviainen A, Kruus K, Suurnäkki A (2013) Cellulose hydrolysis with thermo- and alkali-tolerant cellulases in cellulose-dissolving superbase ionic liquids. RSC Adv 3:20001–20009
Wang Y, Fu Z, Huang H, Yao B, Zhang H, Xiong H, Turunen O (2012) Improved thermal performance of Thermomyces lanuginosus GH11 xylanase by engineering of an N-terminal disulfide bridge. Bioresour Technol 112:275–279
Welton T (1999) Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev 99:2071–2083
Winterhalter C, Liebl W (1995) Two extremely thermostable xylanases of the hyperthermophilic bacterium Thermotoga maritima MSB8. Appl Environ Microbiol 61:1810–1815
Xiong H, Nyyssölä A, Jänis J, Santa H, Pastinen O, von Weymarn N, Leisola M, Turunen O (2004a) Characterization of the xylanase produced by submerged cultivation of Thermomyces lanuginosus DSM 10635. Enzyme Microb Technol 35:93–99
Xiong H, Fenel F, Leisola M, Turunen O (2004b) Engineering the thermostability of Trichoderma reesei endo-1,4-β-xylanase II by combination of disulphide bridges. Extremophiles 8:393–400
Xiong H, von Weymarn N, Turunen O, Leisola M, Pastinen O (2005) Xylanase production by Trichoderma reesei Rut C-30 grown on l-arabinose-rich plant hydrolysates. Bioresour Technol 96:753–759
Zhang J, Zhang H, Wu J, Zhang J, He J, Xiang J (2010) NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids. Phys Chem Chem Phys 12:1941–1947
Zhang T, Datta S, Eichler J, Ivanova N, Axen SD, Kerfeld CA, Chen F, Kyrpides N, Hugenholtz P, Cheng J-F, Sale KL, Simmons B, Rubin E (2011) Identification of a haloalkaliphilic and thermostable cellulase with improved ionic liquid tolerance. Green Chem 13:2083–2090
Zhao H, Jones CL, Baker GA, Xia S, Olubajo O, Person VN (2009) Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J Biotechnol 139:47–54
Acknowledgments
This work was financially supported by the Thailand Research Fund through the Royal Golden Jubilee Ph.D. grant, Contract No. PHD/0189/2548 and Centre of Excellent for Renewable Energy, Chiang Mai University. We thank Johanna Aura for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Huang.
N. Chawachart and S. Anbarasan contributed equally to the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chawachart, N., Anbarasan, S., Turunen, S. et al. Thermal behaviour and tolerance to ionic liquid [emim]OAc in GH10 xylanase from Thermoascus aurantiacus SL16W. Extremophiles 18, 1023–1034 (2014). https://doi.org/10.1007/s00792-014-0679-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-014-0679-0