Abstract
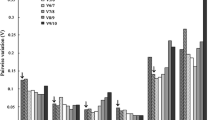
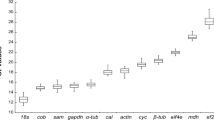
Antarctic ice alga Chlamydomonas sp. ICE-L can endure extreme low temperature and high salinity stress under freezing conditions. To elucidate the molecular acclimation mechanisms using gene expression analysis, the expression stabilities of ten housekeeping genes of Chlamydomonas sp. ICE-L during freezing stress were analyzed. Some discrepancies were detected in the ranking of the candidate reference genes between geNorm and NormFinder programs, but there was substantial agreement between the groups of genes with the most and the least stable expression. RPL19 was ranked as the best candidate reference genes. Pairwise variation (V) analysis indicated the combination of two reference genes was sufficient for qRT-PCR data normalization under the experimental conditions. Considering the co-regulation between RPL19 and RPL32 (the most stable gene pairs given by geNorm program), we propose that the mean data rendered by RPL19 and GAPDH (the most stable gene pairs given by NormFinder program) be used to normalize gene expression values in Chlamydomonas sp. ICE-L more accurately. The example of FAD3 gene expression calculation demonstrated the importance of selecting an appropriate category and number of reference genes to achieve an accurate and reliable normalization of gene expression during freeze acclimation in Chlamydomonas sp. ICE-L.





Similar content being viewed by others
References
Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250
Barsalobres-Cavallari C, Severino F, Maluf M, Maia I (2009) Identification of suitable internal control genes for expression studies in Coffea arabica under different experimental conditions. BMC Mol Biol 10(1):1
Cuevas JC, Lo’pez-Cobollo R, Alca’zar R, Zarza X, Koncz C, Altabella T, Salinas J, Tiburcio AF, Ferrando A (2008) Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiol 148:1094–1105
Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalisation in Arabidopsis. Plant Physiol 139(1):5–17
De Jonge HJ, Fehrmann RS, de Bont ES, Hofstra RM, Gerbens F (2007) Evidence based selection of housekeeping genes. PLoS ONE 2:e898
Dombrowski JE, Martin RC (2009) Evaluation of reference genes for quantitative RT-PCR in Lolium temulentum under abiotic stress. Plant Sci 176:390–396
Exposito-Rodriguez M, Borges AA, Borges-Perez A, Perez JA (2008) Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol 8:131
Gradinger RR (2001) Adaptation of Arctic and Antarctic ice metazoa to their habitat. Zoology 104:339–345
Guenin S, Mauriat M, Pelloux J, Van WO, Bellini C, Gutierrez L (2009) Normalization of qRT-PCR data: the necessity of adopting a systematic, experimental conditions-specific, validation of references. J Exp Bot 60:487–493
Hicks GR, Hironaka CM, Dauvilee D, Funke RP, D’Hulst C, Waffenschmidt S, Ball SG (2001) When simpler is better. Unicellular green algae for discovering new genes and functions in carbohydrate metabolism. Plant Physiol 127:1334–1338
Hong SY, Seo PJ, Yang MS, Xiang F, Park CM (2008) Exploring valid reference genes for gene expression studies in Brachypodium distachyon by real-time PCR. BMC Plant Biol 8:112–123
Hou X, Jiang Y, Li G (2002) Relations of total lipid content and fatty acid composition of Antarctic ice-microalgae to its low temperature adaptability. J Oceanogr Huang Hai Bo Hai Seas 20(1):1–10
Hsiao LL, Dangond F, Yoshida T, Hong R, Jensen RV (2001) A compendium of gene expression in normal human tissues. Physiol Genomics 7:97–104
Huggett J, Dheda K, Bustin S, Zumla A (2005) Real-time RT-PCR normalisation; strategies and considerations. Genes Immun 6:279–284
Iskandar HM, Simpson RS, Casu RE, Bonnett GD, Maclean DJ, Manners JM (2004) Comparison of reference genes for quantitative real-time polymerase chain reaction analysis of gene expression. Plant Mol Biol Rep 22:325–337
Jian B, Liu B, Bi Y, Hou W, Wu C, Han T (2008) Validation of internal control for gene expression study in soybean by quantitative real-time PCR. BMC Mol Biol 9:59
Kan G, Zheng Z, Jiang Y, Miao J, Zhang B, Li G (2006) Salt resistance of Antarctic ice microalga Chlamydomonas sp. J Fishery Sci China 13:73–78
Le Bail A, Dittami S, de Franco PO, Rousvoal S, Cock M, Tonon T, Charrier B (2008) Normalisation genes for expression analyses in the brown alga model Ectocarpus siliculosus. BMC Mol Biol 9:1–75
Liu C, Huang X, Wang X, Zhang X, Li G (2006) Phylogenetic studies on two strains of Antarctic ice algae based on morphological and molecular characteristics. Phycologia 45:190–198
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C[T]) method. Methods 25:402–408
Lovdal T, Lillo C (2009) Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal Biochem 387:238–242
Martin R, Hollenbeck V, Dombrowski J (2008) Evaluation of reference genes for quantitative RT-PCR in Lolium perenne. Crop Sci 48:1881–1887
Mock T, Thomas DN (2005) Recent advances in sea-ice microbiology. Environ Microbiol 7:605–619
Morgan-Kiss RM, Priscu JC, Pocock T, Gudynaite-Savitch L, Huner NPA (2006) Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol Mol Biol Rev 70:222–252
Nicot N, Hausman J, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe A, Hattori R (eds) Culture and collections of algae. Proc. U.S.-Japan Conference, Hakone
Reid KE, Olsson N, Schlosser J, Peng F, Lund ST (2006) An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol 6:27
Rosic N, Pernice M, Rodriguez-Lanetty M, Hoegh-Guldberg O (2010) Validation of housekeeping genes for gene expression studies in Symbiodinium exposed to thermal and light stress. Mar Biotech 29:1–11
Udvardi MK, Czechowski T, Scheible WR (2008) Eleven golden rules of quantitative RT-PCR. Plant Cell 20:1736–1737
Upchurch RG (2008) Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotech Lett 30:967–977
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034
Vicentini R, Sassaki FT, Gimenes MA, Maia IG, Menossi M (2005) In silico evaluation of the Eucalyptus transcriptome. Gene Mol Biol 28:487–495
Wu G, Liu C, Liu S, Cong B, Huang X (2010) High-quality RNA preparation for cDNA library construction of the Antarctic sea–ice alga Chlamydomonas sp. ICE-L. J Appl Phycol 22(6):779–783
Zhang Z, Hu J (2007) Development and validation of endogenous reference genes for expression profiling of Medaka (Oryzias latipes) exposed to endocrine disrupting chemicals by quantitative real-time RT-PCR. Toxicol Sci 95:356–368
Zhang P, Liu S, Cong B, Wu G, Liu C, Lin X, Shen J, Huang X (2010) A novel omega-3 fatty acid desaturase involved in acclimation processes of polar condition from Antarctic ice Algae Chlamydomonas sp. ICE-L. Mar Biotechnol 13:393–401
Acknowledgments
This work was financially supported by a grant of National Natural Science Foundation (40876106) for Chenlin Liu.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Huang.
Rights and permissions
About this article
Cite this article
Liu, C., Wu, G., Huang, X. et al. Validation of housekeeping genes for gene expression studies in an ice alga Chlamydomonas during freezing acclimation. Extremophiles 16, 419–425 (2012). https://doi.org/10.1007/s00792-012-0441-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-012-0441-4