Abstract
Objective
This cross-sectional study investigated the association between fungiform papillae (FP) numbers and tooth number anomalies in children, considering variables related to hypodontia and hyperdontia. The aim was to explore this association while adjusting for age and sex differences.
Materials and methods
A total of 144 children (aged 8–10) were categorized into hypodontia (n = 48), hyperdontia (n = 48), and control groups (n = 48). Clinical and radiographic diagnoses were used to classify tooth number anomalies. Hypodontia was categorized by number and location, while hyperdontia was categorized by number, shape, and location. FP were assessed using the Denver Papillae Protocol. Data analyses were performed using NCSS software, with p < 0.05 considered statistically significant.
Results
The hypodontia group (22.5 ± 8.4) exhibited significantly lower FP than the control group (30.4 ± 9.2) and the hyperdontia group (27.9 ± 7.8) (p < 0.0005, p = 0.003, respectively). No significant difference existed between the hyperdontia and control groups. FP numbers in hypodontia subgroups showed no significant differences based on teeth agenesis numbers or locations. Similarly, hyperdontia subgroup analyses revealed no significant differences in FP numbers based on supernumerary teeth shapes (supplemental, conical, tuberculoid, paramolar) or the numbers of supernumerary teeth.
Conclusions
The lower FP numbers in children with hypodontia suggested an association between teeth and FP number. However, the non-significant difference in FP numbers with hyperdontia underscored the complexity of tooth development, warranting further investigations.
Clinical relevance
Children with hypodontia may exhibit distinct FP numbers compared to those without tooth number anomalies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tooth development is a sophisticated process driven by intricate interactions between the oral epithelium and the ectomesenchyme. The oral epithelium originates from the ectoderm, and the ectomesenchyme is derived from the neural crest [1]. This process involves a complex interplay of signalling centers, transcription factors, and pathways [1, 2]. Notably, teeth and taste buds, being neurosensory organs, share a common developmental origin rooted in the interactions of epithelial and progenitor cells [3]. The development of structures like teeth, hair, glands, intestinal villi, and fungiform papillae (FP), vital for taste perception [4], involves analogous epithelial-mesenchymal interactions [5,6,7,8]. Crucial signaling pathways are engaged in the formation of teeth and tongue papillae [9, 10]. Animal studies have revealed shared genes influencing the development of both taste buds and teeth. For instance, Bloomquist et al. [3] linked tooth and taste bud density through Wingless signals, also identifying signals that distinguish organ identity from a common epithelial lamina using BMP and Hedgehog signaling. Recognizing these developmental parallels between teeth and taste papillae offers insights into the etiology of dental anomalies, including hypodontia.
Hypodontia, characterized by the reduction in tooth number due to the absence of one or more tooth germs, stands as one of the most common developmental anomalies [11, 12]. Its prevalence ranges from 3 to 10%, depending on the study population. It predominantly affects the permanent dentition, excluding third molars, and shows a slight female-to-male predilection of about one-and-a-half to one [11]. In the Caucasian population, Soxman [11] reported that 80% of individuals with hypodontia exhibited only one or two agenesis. In Turkey, the prevalence of hypodontia in the permanent dentition was reported to be 6.02% [13], similar to that found in the Slovenian population, which is considered part of Central Europe, where a prevalence of 6.9% was reported [14].
Hyperdontia, characterized by an excess number of teeth in the dentition, exhibits ethnic variations, with a prevalence ranging from 0.1% to 3.8% in Caucasians and slightly higher rates in Asian populations [15]. In the Turkish population, the prevalence of hyperdontia was found to be 0.5% [16]. Males show a two-to-one predominance over females in cases of hyperdontia. Single-tooth hyperdontia is reported in approximately 80% of cases, primarily observed in the permanent dentition, with about 95% of these cases affecting the maxillary arch, particularly in the anterior region [11].
Previous studies have explored the relationship between the number of FP and various factors such as taste perception, taste sensitivity, body mass index, age, gender, different habits, and diseases [4, 17,18,19,20]. However, to date, no study has investigated the relationship between the FP number and orodental abnormalities in humans. This study aimed to investigate the association between the FP number in children with hypodontia or hyperdontia. The null hypotheses were as follows: 1) There would be no significant difference in the FP number between the control and hypodontia groups or between the control and hyperdontia groups. 2) There would be no association between the FP number and variables related to teeth agenesis, including their quantity and location. 3) There would be no association between the FP number and variables related to supernumerary teeth, including their quantity, location, and shapes.
Material and methods
Subjects
We recruited healthy children who presented to the Department of Pediatric Dentistry at Marmara University Faculty of Dentistry, Istanbul, Turkey for dental and radiographic examinations. The study was conducted in accordance with Declaration of Helsinki and was approved by Marmara University Clinical Research Ethics Committee (protocol #2016–62). The parents of each participant signed the volunteer information & consent forms.
Subjects were required to meet specific criteria outlined in the inclusion criteria:
-
Healthy children with no systemic diseases.
-
Radiographs confirming the presence of hypodontia or hyperdontia.
-
Age between 8 and 10 years.
Exclusion Criteria:
-
Recent history of drug use associated with infections or surgeries within the last month.
-
Patients exhibiting a severe gagging reflex or those who experienced issues during tongue drying and dyeing.
-
History of premolar extraction according to the parents in the related region.
A control group of healthy children aged between 8 and 10 years, consisting of an equal distribution of 50% girls and 50% boys, without any tooth number anomalies, was selected for comparison.
Sample size calculation
The sample size calculation was conducted using the online Power calculator for binary outcome equivalence trial available at https://www.sealedenvelope.com/power/binary-equivalence/. The calculation was based on specific parameters: an alpha level of 0.05, a power of 80% (1-beta), a success rate of 90% in both groups, and an equivalence limit of 18%. These parameters were chosen to control both Type I and Type II errors effectively. The calculated sample size indicated that a total of 144 patients were required, with 48 individuals assigned to each group.
Study design
The medical and dental histories of the participants were recorded along with their demographic data (name, gender, place of birth, date of birth). Subsequently, visual dental and radiological examinations were conducted for each child. Comprehensive records were maintained for each child, documenting the specific details of hypodontia (number and location of missing teeth) and hyperdontia (number, location, and shape of supernumerary teeth). The tongues were stained, post-procedure photographs were taken, and then FP were counted following the Denver Papillae Protocol [21]. The study was conducted from March 2016 to October 2017, and the data were carefully labelled, registered, and analysed. All patients underwent a re-evaluation in the subsequent year, involving the examination of the new panoramic radiographs to assess treatment effectiveness and provide necessary follow-up every 6 months.
Identification of hypodontia/ hyperdontia
In cases of missing teeth, the patients' dental histories were meticulously investigated with input from their parents. To diagnose tooth agenesis, panoramic radiographs were taken for all patients, and periapical radiographs were selectively used in cases with artifacts or image quality concerns in the panoramic radiographs. This combined approach allowed us to record the number and location of any missing teeth in the anterior or posterior regions of the upper and lower jaws while adhering to the As Low As Reasonably Achievable (ALARA) principle. Teeth agenesis were classified by location as anterior, posterior and anterior + posterior [11]. Hypodontia group was also categorized into three subgroups: 1 to 2 teeth agenesis; 3 to 5 teeth agenesis; and more than 6 teeth agenesis.
We diagnosed clinically erupted supernumerary teeth during the oral examination. The unerupted supernumerary teeth were detected radiographically. The surgical treatment involved the removal of unerupted supernumerary teeth and/or bonding an attachment to the underneath permanent tooth. The surgical procedure was performed either locally or under general anesthesia. Cone-beam computed tomography (CBCT) images were obtained when panoramic or periapical X-rays could not adequately display the precise location of the supernumerary teeth. We recorded tooth types according to their morphologies as supplemental or rudimentary. Rudimentary teeth were classified as conical (peg-shaped), tuberculoid (barrel-shaped) in the anterior with one or more cusps, and paramolar (small premolar or molar-like in shape/molariform) [11]. Hyperdontia group was also categorized into three subgroups: one supernumerary tooth; double supernumerary teeth; and more than two supernumerary teeth.
Obtaining the images of tongue and determination of FP numbers
The Denver Papillae Protocol was used to take pictures of the tongue and determine the FP number. The researchers showed the children an educational video in which they painted each other's tongue and took photographs. The researcher (BA) demonstrated how to pose for the photographs. Before painting the tongue, the researchers ensured that the children could place their hands under their chin in an appropriate position and keep their tongue stabilized (Fig. 1). The researcher (BA) dried the tongue with a paper towel and applied one or two drops of 1/36 diluted blue food dye (Brilliant Blue FCF, Sensient Technologies Europe GmbH, Geesthacht, Germany). A circle with a diameter of 10 mm was cut from filter paper and placed on the left side of the midline of the tongue. The children were instructed to stick out their tongue and hold it between their teeth. Then, photographs were taken using a digital compact camera (Nikon COOLPIX P610, Tokyo, Japan). As Denver papillae protocol suggested, training was performed via https://www.jove.com/v/52860/denver-papillae-protocol-for-objective-analysis-of-fungiform-papillae. The researchers (BA, EEK) independently scored each photograph using ImageJ software (version 1.46r, 32-bit). ImageJ facilitates the display, editing, analysis, processing, storage, and printing of images in various bit depths, including 8-bit, 16-bit, and 32-bit. During the scoring process, if the difference between the two FP raw scores exceeded 10%, both scored photos were reviewed together. If consensus was still not reached, a third researcher's opinion (AM) was sought to resolve discrepancies [21].
Statistical evaluation
The Kolmogorov–Smirnov test was used to determine whether continuous variables were normally distributed. Descriptive statistics were presented as frequencies and percentages for the sex variable and mean and standard deviation for the age and FP number variable. The association between the study groups (hypodontia, hyperdontia, control) and sex was evaluated using the chi-square test. The association between the study groups and age was also evaluated using the one-way analysis of variance (ANOVA) test followed by Tukey's multiple comparison test for subgroup analysis. Multivariate linear regression model was fitted for the FP number variable. This model included: study groups (type), sex, and age. Data analysis was performed using NCSS (Number Cruncher Statistical System) 2007 Statistical Software (Utah, USA). The significance level was set at p < 0.05.
Results
Initially, 156 children (52 per group) were screened for eligibility. Among them, four patients in the hypodontia group developed teeth in the years 2017 and 2018, resulting in a reduction of the hypodontia group to 48 children. To maintain uniformity, eight children (2 boys and 2 girls from each group) were randomly excluded from both the hyperdontia and control groups (n = 8). This resulted in 144 children confirmed as eligible and included in the study, comprising 48 with hypodontia (18 boys, 30 girls), 48 with hyperdontia (31 boys, 17 girls), and 48 without any tooth number anomalies (24 boys, 24 girls). The intraclass correlation coefficient for FP number measurements demonstrated high consistency among researchers within specific group; control group, hypodontia group, hyperdontia group, and the entire study cohort (0.99, 95% CI: 0.98–0.99).
FP counts were based on the data collected by one observer (BA) to ensure consistency. Examination of participant demographics revealed no significant age differences between groups (Table 1). The mean FP number for boys was 26.9 ± 8.5, and for girls, it was 25.9 ± 9.6. Importantly, there was no statistically significant difference in mean FP numbers between boys and girls (p = 0.46). In terms of FP distribution, counts ranged from 9 to 55 papillae within a 0.75 cm2 area, with an overall mean of 26.9 ± 9.1 (Table 2). The effects of age and sex parameters on FP numbers were not significant (p > 0.05) whereas hypodontia, hyperdontia and control groups were significant (p = 0.001) when using multivariate linear regression analysis (Table 2).
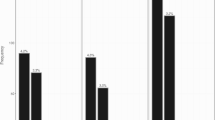
The mean FP number in the hypodontia group was found to be 22.5 ± 8.4, while in the hyperdontia group, it was 27.9 ± 7.8. In the control group, the mean FP number was 30.4 ± 9.2 (Table 2). A statistically significant difference was observed among the groups (p < 0.0005). Specifically, the mean FP number in the hypodontia group was significantly lower than that in both the control group (p < 0.0001) and the hyperdontia group (p = 0.003). However, there was no statistically significant difference in the mean FP numbers between the control and hyperdontia groups (p > 0.05) (Table 2).
Hypodontia group evaluation
Among the 48 children with hypodontia, a total of 186 cases of tooth agenesis were observed. Upon examining the distribution of tooth agenesis by region, it was found that 42% (n = 22) occurred in the anterior region, 38% (n = 16) were limited to the posterior region, and 20% (n = 10) affected both the anterior and posterior regions. The mean number of FP in the anterior, posterior, and anterior + posterior subgroups were 23.5 ± 8.4, 23.9 ± 8.6, and 20.1 ± 7.8, respectively (SI Table 1). Statistical analysis revealed no significant difference in the teeth agenesis number among the anterior, posterior, and anterior + posterior subgroups in the hypodontia group (p = 0.47) (SI Table 1). However, it is worth noting that patients with tooth agenesis in both the anterior and posterior regions had the fewest missing teeth.
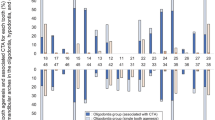
The mean FP number for subgroups with 1 to 2 teeth agenesis, 3 to 5 teeth agenesis, and more than 6 teeth agenesis were 24.5 ± 8.5, 22.1 ± 7.5, and 19.5 ± 7.7, respectively (SI Table 2). These findings suggested a pattern of decreasing FP numbers with an increasing number of tooth agenesis (Fig. 2). However, it's important to note that our analysis did not reveal statistical significance.
Distribution of FP numbers according to tooth number anomalies. Agenesis_to_Supernumerary scale; 0 represented control group meaning no supernumerary teeth or tooth agenesis in children. Lower than 0 down to -20 showed the number of tooth agenesis in children. Higher than 0 up to 5 showed the number of supernumerary teeth in children. Fungiform_Papillae_Number scale; 0 up to 60 represented the fungiform papillae number within a circle with a diameter of 10 mm
Hyperdontia group evaluation
A total of 70 supernumerary teeth were identified in the hyperdontia group. Among them, 10% (n = 7) were supplemental, 45% (n = 32) were conical, 28% (n = 20) were tuberculoid, and 14% (n = 11) were paramolar teeth. When examining the mean FP number according to the tooth types in the hyperdontia group, the highest FP number was observed in patients with paramolar teeth (30.3 ± 8.6), followed by conical teeth (28.2 ± 8.3), supplemental teeth (26.2 ± 9.1), and tuberculoid teeth (24.8 ± 6.9). However, there were no significant differences between the subgroups (p = 0.46) (SI Table 3).
In the hyperdontia group, 73% of the patients had one supernumerary tooth (n = 35), 18% had double supernumerary teeth (n = 9), and 8% had more than two supernumerary teeth (n = 4). The mean number of FP in these subgroups was 27 ± 8.4, 28.2 ± 6.6, and 25.8 ± 6.7, respectively (Fig. 2) (SI Table 4). There was no significant difference or relationship observed between the number of supernumerary teeth and the FP number (p = 0.86) (SI Table 4).
Discussion
We conducted this study to explore the relationship between the number of FP and tooth anomalies in humans. Drawing inspiration from Bloomquist et al. [3], which focused on cichlid fish, our study is among the first to investigate this association in humans. On the other hand, Wong et al. [22] investigated the effects of tooth agenesis on bitter taste perception, our research specifically delves into the association between the number of teeth and FP in humans.
Various methods have been used to determine the number of FP. Miller et al. [23] initially counted papillae on human cadaver tongues. Subsequent studies in live humans involved painting the tongue with food dye and capturing tongue images with a video-microscope [24,25,26,27]. However, this method proved time-consuming, and a digital camera was later employed as a more efficient alternative [27]. Previous studies have developed an algorithm to count FP in tongue images, replacing the traditional manual counting method [28, 29]. Various computer systems or software are necessary for determining FP count; further research is needed to confirm their accuracy.
The Denver Papilla Protocol, developed by Nuessle et al. [21], is a currently popular method for FP counting as it requires less time, tolerates tongue and head movement better, and is cost-effective. Another current method for FP counting involves the use of a confocal laser scanning microscope, which can also determine the number of taste buds, but it is costly and requires expert knowledge [30]. In a study similar to ours, a group of children aged 8–11 years were painted with blue food dye and photographed with a digital camera to determine FP count [31]. Similarly, a study on 101 children aged 11–15 years also used blue food dye and investigated the relationship between FP count and taste sensitivity [32]. Other recent studies have similarly utilized the Denver Papilla Protocol method to assess FP count [33,34,35]. We excluded two individuals with a recent history of medication use within the last month, as such factors can potentially influence FP morphology, as evidenced by Piochi et al. [36]. We experienced difficulties while taking tongue photos from these young children. Three children with severe gagging reflex and two children with inadequate tongue drying and dyeing were dismissed. Additionally, one patient with a reported history of unknown extraction in the relevant premolar region, as indicated by the parents, was excluded to eliminate any bias associated with hypodontia.
The relationship between FP number and age has been a subject of investigation in various cross-sectional studies. For instance, Correa et al. [37] examined changes in FP density across different age groups, including children aged 7–8 years, 9–10 years, 11–12 years, and adults aged 20–24 years. While they found no statistically significant difference in the number of FP between children in the 7–8 years age group and those in other age groups, they observed that children in this particular age group had a higher number of FP compared to adults. Another study on 83 children aged 8–11 years showed a negative correlation between age and the number of FP, indicating a decrease with age [31]. In our previous cross-sectional study involving children aged 5–10 years, a decrease in FP numbers with age was reported [33]. This observation suggested a potential association between FP number and age within the specific age range. Therefore, our present study targeted the narrow age range of 8–10 years to minimize the potential confounding effect of age on FP number. Remarkably, a study involving a wide age range (21–84) showed that with every 5-year increase in age groups, the mean density of FP decreased by 2.8 papillae/cm2 [38]. However, it is noteworthy that studies in adult populations have reported mixed results concerning the relationship between FP number and age, with some finding no significant differences. For instance, Bajec and Pickering [39] observed no significant differences in FP number across age groups in adults. Similarly, Feeney and Hayes [40] and Masi et al. [41] found no significant associations between age and FP number in their studies.
Numerous cross-sectional studies have explored the association between sex and the quantity of FP. In our study, we investigated this relationship and found no statistically significant difference in the number of FP between males and females, consistent with previous studies [31, 37, 39, 41]. Jilani et al. [31] reported similar findings, with the range between 14 and 46 in a 6 mm diameter circle. Additionally, Sobek and Jagielski [32] found no significant disparity in FP numbers between boys and girls in children aged 11–15 years, with a median of 27 and a range from 12 to 47. Correa et al. [37] and Fogel and Blissett [42] also reported no significant sex difference in FP numbers in studies with children. Fogel and Blissett's study [42], for instance, found that the mean number of FP counted was 37.3 ± 9.9 with a density ranging between 23–67/cm2. Kalaoglu et al. [33] found that girls had higher FP numbers than boys aged 5–10 years. On the other hand, studies involving larger adult populations have produced differing results. Fischer et al. [38] reported that females had more FP than males in a study with 2371 adult participants. These contrasting findings suggest that the relationship between FP numbers and sex may be influenced by various factors such as age, ethnicity, and environmental conditions [36,37,38]. Further research is needed to better understand the FP numbers, considering various factors that may contribute to the observed differences.
The primary limitation of this cross-sectional study design lies in its simultaneous assessment of FP number and tooth number anomalies, as it does not provide evidence of a temporal relationship between exposure and outcome. Since FP number can change over time, a deeper understanding would require a longitudinal study. Future research could greatly benefit from repeated assessments over time and expansion to different age groups, although challenges in assessing the papillae of younger children should be considered. Despite these limitations, we believe this study is crucial as the first investigation on this subject, contributing to the detection of a relationship between fungiform papilla number and tooth number.
This research was centered around comparing two different tissues with similar phenotypes and developmental delays. The significance of this study lies in the observation that the FP numbers is reduced in cases of hypodontia. The hyperactivity of dental lamina is the most acceptable and widely accepted theory for supernumerary teeth. Lu et al. [43] mentioned that it is unclear whether the hyperactivity of the dental lamina, which leads to hyperdontia, particularly in non-syndromic cases, has a genetic or environmental origin. Our study did not identify any significance in cases of hyperdontia, indicating the need for more comprehensive investigations on this subject. Future investigations could explore the specific mechanisms linking tooth development anomalies and taste bud alterations and guiding personalized approaches for individuals with dental anomalies.
In conclusion, our study revealed a statistically significant reduction in the average number of FP in the hypodontia group compared to both the hyperdontia and control groups, in line with our initial hypotheses. However, the absence of a statistically significant difference between the control and hyperdontia groups underscored the intricate relationships between tooth and papillae numbers, prompting further investigation. The null hypotheses concerning the quantity, location, and shape of tooth number anomalies were accepted, as no associations were discovered between the FP number and variables associated with teeth agenesis, or related to supernumerary teeth. These findings not only emphasized the complexity of these relationships but also served as a launching point for future investigations.
Data availability
The entirety of the data analyzed in this study has been included in the published article. Upon making a reasonable request, the corresponding author will provide access to the raw data.
References
Zhang H, Gong X, Xu X, Wang X, Sun Y (2023) Tooth number abnormality: from bench to bedside. Int J Oral Sci 15(1):5
Brook A, Jernvall J, Smith R, Hughes T, Townsend G (2014) The dentition: the outcomes of morphogenesis leading to variations of tooth number, size, and shape. Aust Dent J 59:131–142. https://doi.org/10.1111/adj.12160
Bloomquist RF, Parnell RF, Phillips KA, Fowler TE, Yu TY, Sharpe PT, Streelman JT, Shubin NH (2015) Coevolutionary patterning of teeth and taste buds. Proc Natl Acad Sci USA 112:E5954–E5962
Dinnella C, Monteleone E, Piochi M, Spinelli S, Prescott J, Pierguidi L, Gasperi F, Laureati M, Pagliarini E, Predieri S, Torri L, Barbieri S, Valli E, Bianchi P, Braghieri A, Caro AD, di Monaco R, Favotto S, Moneta E (2018) Individual variation in PROP status, FP density, and responsiveness to taste stimuli in a large population sample. Chem Senses 43:697–710
Kondo S, Miura T (2011) Reaction-diffusion model as a framework for understanding biological pattern formation. Science 329(5999):1616–1620
Sick S, Reinker S, Timmer J, Schlake T (2006) Wnt and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 314(5804):1447–1450
Mou C, Pitel F, Gourichon D, Vignoles F, Tzika A, Tato P, ... Headon DJ (2011) Cryptic patterning of avian skin confers a developmental facility for the loss of neck feathering. PLoS Biol 9(3):e1001028. https://doi.org/10.1371/journal.pbio.1001028
Chou SW et al (2011) Interactions between Shh, Sostdc1, and Wnt signaling and a new feedback loop for spatial patterning of the teeth. Development 138(9):1807–1816
Liu F, Millar SE (2010) Wnt/β-catenin signaling in oral tissue development and disease. J Dent Res 89(4):318–330. https://doi.org/10.1177/0022034510363373
Sagai T, Amano T, Maeno A et al (2017) SHH signaling directed by two oral epithelium-specific enhancers controls tooth and oral development. Sci Rep 7:13004. https://doi.org/10.1038/s41598-017-12532-y
Soxman JA, Barsamian P, Wunsch CM, & Haberland AM (2019) Anomalies of the developing dentition: a clinical guide to diagnosis and management (Section 4). Springer.https://doi.org/10.1007/978-3-030-03164-0
Fekonja A (2013) Comparison of mesiodistal crown dimension and arch width in subjects with and without hypodontia. J Esthet Restor Dent 25(3):203–210. https://doi.org/10.1111/jerd.12026
Cantekin K, Dane A, Miloglu O, Kazanci F, Bayrakdar S, Celikoglu M (2012) Prevalence and intra-oral distribution of agenesis of permanent teeth among Eastern Turkish children. Eur J Paediatr Dent 13:53–56
Fekonja A (2015) Jan-Feb) Hypodontia prevalence over four decades in a Slovenian population. J Esthet Restor Dent 27(1):37–43. https://doi.org/10.1111/jerd.12076
Pinkham JR, Cassamassimo PS, Fields JH, McTigue DM, Nowak A (2013) Pediatric Dentistry: Infancy Through Adolescence (5th ed.). Elsevier Health Science
Gurbuz O, Ersen A, Dikmen B, Gumustas B, Gundogar M (2019) The prevalence and distribution of dental anomalies in the Turkish population. J Anat Soc India 68(1):46–51. https://doi.org/10.4103/JASI.JASI_31_19
Bakke A, Vickers Z (2011) Effects of bitterness, roughness, PROP taster status, and FP density on bread acceptance. Food Qual Prefer 22(4):317–325
Segovia C, Hutchinson I, Laing DG, Jinks AL (2002) A quantitative study of FP and taste pore density in adults and children. Dev Brain Res 138:135–146
Walliczek-Dworschak U, Schöps F, Feron G, Brignot H, Hähner A, Hummel T (2017) Differences in the density of FP and composition of saliva in patients with taste disorders compared to healthy controls. Chem Senses 42(8):699–708. https://doi.org/10.1093/chemse/bjx054
Melis M, Mastinu M, Naciri LC, Muroni P, Barbarossa I (2022) Associations between Sweet Taste Sensitivity and Polymorphisms (SNPs) in the TAS1R2 and TAS1R3 Genes, Gender, PROP Taster Status, and Density of FP in a Genetically Homogeneous Sardinian Cohort. Nutrients. https://doi.org/10.3390/nu14224903
Nuessle TM, Garneau NL, Sloan MM, Santorico SA (2015) Denver papillae protocol for objective analysis of FP. J Vis Exp 100:e52860
Wong SW, Han D, Zhang H et al (2018) Nine novel PAX9 mutations and a distinct tooth agenesis genotype-phenotype. J Dent Res 97(2):155–162. https://doi.org/10.1177/0022034517729322
Miller IJ (1988) Human taste bud density across adult age groups. J Gerontol 43(1):B26–B30
Miller IJ Jr, Reedy FE Jr (1990) Quantification of FP and taste pores in living human subjects. Chem Senses 15(3):281–294
Schiffman SS, Graham BG, Suggs MS, Sattely-Miller EA (1998) Effect of psychotropic drugs on taste responses in young and elderly persons. Ann N Y Acad Sci 855:732–737
Sollars SI, Bernstein IL (2000) Neonatal chorda tympani transection permanently disrupts fungiform taste bud and papilla structure in the rat. Physiol Behav 69:439–444
Shahbake M, Hutchinson I, Laing DG, Jinks AL (2005) Rapid quantitative assessment of FP density in the human tongue. Brain Res 1052(2):196–201
Valencia E, Rios H, Verdalet I, Hernandez J, Juarez S, Herrera R, Silva ER (2016) Automatic counting of FP by shape using cross-correlation. Comput Math Methods Med 76:168–172
Sanyal S, O’Brien SM, Hayes JE, Feeney EL (2016) TongueSim: Development of an automated method for rapid assessment of FP density for taste research. Chem Senses 41:357–365
Saito T, Ito T, Ito Y, Yamada T, Okamoto M, Manabe Y (2016) Gustatory dysfunction and decreased number of fungiform taste buds in patients with cholesteatoma. Ann Otol Rhinol Laryngol 125(9):704–709
Jilani H, Ahrens W, Buchecker K, Russo P, Hebestreit A (2017) Association between the number of FP on the tip of the tongue and sensory taste perception in children. Food Nutr Res 61(1):1348865
Sobek G, Jagielski P (2022) The number of FP, taste sensitivity, and smell functions of children aged 11–15. Nutrients 14(13):2578. https://doi.org/10.3390/nu14132578
Kalaoglu EE, Yazici B, Mentes A (2021) Relationship between the FP number and dental caries in primary teeth: a cross-sectional study. Clin Oral Invest 25(12):6931–6937. https://doi.org/10.1007/s00784-021-03983-9
Speth US, Kluwe L, Gosau M, Friedrich RE (2023) Relative size of FP in patients with neurofibromatosis Type 1. J Stomatol Oral Maxillofac Surg 124(3):101394
Dinnella C, Napolitano F, Spinelli S, Monteleone E, Pacelli C, Braghieri A (2023) Factors affecting stated liking for meat products: focus on demographics, oral responsiveness, personality, and psycho attitudinal traits. Meat Sci 195:109004
Piochi M, Dinnella C, Prescott J, Monteleone E (2018) Associations between human FP and responsiveness to oral stimuli: effects of individual variability, population characteristics, and methods for papillae quantification. Chem Senses 43(5):313–327. https://doi.org/10.1093/chemse/bjy015
Correa M, Hutchinson I, Laing DG, Jinks AL (2013) Changes in FP density during development in humans. Chem Senses 38:519–527
Fischer ME, Cruickshanks KJ, Schubert CR, Pinto A, Klein R, Pankratz N, Pankow JS, Huang GH (2013) Factors related to FP density: the beaver dam offspring study. Chem Senses 38:669–677
Bajec MR, Pickering GJ (2008) Thermal taste, PROP responsiveness, and perception of oral sensations. Physiol Behav 95(4):581–590
Feeney EL, Hayes JE (2014) Regional differences in suprathreshold intensity for bitter and umami stimuli. Chemosens Percept 7:147–157
Masi C, Dinnella C, Monteleone E, Prescott J (2015) The impact of individual variations in taste sensitivity on coffee perceptions and preferences. Physiol Behav 138:219–226. https://doi.org/10.1016/j.physbeh.2014.10.031
Fogel A, Blissett J (2017) Past exposure to fruit and vegetable variety moderates the link between FP density and current variety of FV consumed by children. Physiol Behav 177:107–112
Lu X, Yu F, Liu J, Cai W, Zhao Y, Zhao S, Liu S (2017) The epidemiology of supernumerary teeth and the associated molecular mechanism. Organogenesis 13(3):71–82. https://doi.org/10.1080/15476278.2017.1332
Acknowledgements
The authors are thankful to all volunteers for joining this study. Rana Konyalıoglu who performed all statistical analyses is appreciated for working for this study.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). No funds or grants was received.
Author information
Authors and Affiliations
Contributions
Belgin Alp: Conceptualization, Data Curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Data curation, Writing-original draft. Elif Ece Kalaoglu: Conceptualization, Data Curation, Formal analysis, Resources, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing- original draft, Writing-Review & Editing. Ali Mentes: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing-Review & Editing. All authors have reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with Declaration of Helsinki and was approved by Marmara University Clinical Research Ethics Committee (protocol #2016–62). The parents of each participant signed the volunteer information & consent forms.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The work was conducted in Marmara University Dentistry Faculty Department of Pediatric Dentistry.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alp, B., Kalaoglu, E.E. & Mentes, A. Investigation of the fungiform papillae number in children with tooth number anomalies. Clin Oral Invest 28, 297 (2024). https://doi.org/10.1007/s00784-024-05696-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05696-1