Abstract
Objectives
Observational studies suggested an inverse association between physical activity and periodontitis. However, observational studies might be subject to unobserved confounding and reverse causation bias. We conducted an instrumental variable study to strengthen the evidence on the relationship between physical activity and periodontitis.
Materials and methods
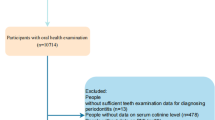
We used genetic variants associated with self-reported and accelerometer-assessed physical activity in 377,234 and 91,084 UK Biobank participants, respectively, as instruments. For these instruments, genetic associations with periodontitis were obtained from 17,353 cases and 28,210 controls in the GeneLifestyle Interactions in Dental Endpoints consortium.
Results
We found no evidence for effects of self-reported moderate-to-vigorous physical activity, self-reported vigorous physical activity, accelerometry “average accelerations,” and “fraction of accelerations > 425 milli-gravities” on periodontitis. For example, the odds ratio for self-reported moderate-to-vigorous physical activity was 1.07 (95% credible interval: 0.87; 1.34) in Causal Analysis using Summary Effect Estimates. We conducted sensitivity analyses to rule out weak instrument bias and correlated horizontal pleiotropy.
Conclusions
The study does not support an effect of physical activity on the risk of periodontitis.
Clinical relevance
This study provides little evidence that recommending physical activity would help prevent periodontitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic conditions, including obesity and diabetes, and inflammatory conditions such as rheumatic diseases are linked to periodontitis [1, 2]. Physical inactivity is considered a modifiable risk factor for metabolic and inflammatory disease development and progression [3, 4]. Meta-analysis of observational data strongly suggests that physical activity is prospectively related to a lower incidence of diabetes [5]. Regular physical activity can reduce diabetes risk by enhancing energy expenditure, insulin action through increased muscle glucose uptake, and muscle insulin sensitivity [6]. The observational literature further shows that physical activity is associated with reduced risk of rheumatoid arthritis [7]. Modulation of inflammation is one of the postulated mechanisms behind the apparent relationship between physical activity and rheumatic arthritis [6]. Genetically instrumented physical activity was further found to be associated with fewer lymphocytes and eosinophils, suggesting a potential effect of physical activity in improving the inflammatory state [8].
Several studies have also suggested an inverse association between physical activity and periodontitis [9, 10]. However, the available studies on physical activity and periodontitis are observational. Observational studies are vulnerable to various biases such as confounding (low physical activity may correlate with other periodontitis risk factors) or reverse causation (symptoms of periodontitis may result in reduction of physical activity). Confounding by measured factors can be adjusted for using approaches such as multivariable regression, propensity score matching, and inverse probability weighting [11]. The validity of the estimates obtained from such methods relies on the assumption that all confounders have been measured and adjusted for. These potential biases may limit the validity of findings derived from traditional observational research [12]. Instrumental variable analysis is an approach to obtain unbiased inference even in the presence of unobserved confounders and reverse causation [13, 14]. We employed genetic variants from genome-wide association studies (GWAS) of physical activity traits as instruments to examine the effect of self-reported and accelerometer-assessed physical activity on the risk of periodontitis in a two-sample instrumental variable study.
Materials and methods
Genotype data for physical activity
We used GWAS-level genotyping data from a GWAS of self-reported and accelerometer-based physical activity conducted in the UK Biobank [15] (Table 1). UK Biobank is a community-based prospective cohort study that recruited over 500,000 men and women ages 40–69 [16]. Self-reported levels of physical activity were ascertained in 377,234 UK Biobank participants using the International Physical Activity Questionnaire Short Form [17]. Moderate-to-vigorous physical activity (MVPA) was computed by taking the sum of total minutes per week of moderate and vigorous physical activity multiplied by eight, corresponding to their metabolic equivalents [15]. Individuals who met 3 or more days per week of vigorous physical activity (VPA) were classified as vigorously physical active [15].
For objective assessment of physical activity, a subset of 103,712 participants wore an Axivity AX3 triaxial accelerometer on the wrist for a 7-day period between 2013 and 2015 [18]. After calibration, removal of gravity and sensor noise, and identification of wear/nonwear episodes, the remaining 100 Hz raw triaxial acceleration data was used to calculate physical activity variables. For the GWAS [15], “average acceleration” (AVEACC) (in milli-gravities (mg)) was used as the exposure variable derived from accelerometer wear. GWAS of fraction of accelerations > 425 mg (ACC425) was also derived because it corresponds to an equivalent of VPA (six metabolic equivalent of tasks (MET)) [19].
Genetic associations with periodontitis
Genetic association estimates for periodontitis of instruments for physical activity traits were obtained from a GWAS of European studies contributing to the GeneLifestyle Interactions in Dental Endpoints (GLIDE) consortium, totaling 17,353 clinical periodontitis cases and 28,210 controls (Table 1) [20]. Periodontitis cases were classified by either the Centers for Disease Control and Prevention/American Academy of Periodontology or the Community Periodontal Index case definition.
Power calculation
Power calculations were performed separately for each physical activity-periodontitis combination in inverse variance weighted (IVW) analyses (see “Statistical analysis”) according to [21]. Given α = 5%, we had ≥ 80% power when the expected odds ratios (OR) for periodontitis were ≤ 0.91, ≤ 0.89, ≤ 0.87, ≤ 0.80 for MVPA, VPA, AVEACC, and ACC425, respectively.
Statistical analysis
We used two-sample instrumental variable estimation based on GWAS summary statistics to estimate effects of genetically instrumented physical activity exposures on periodontitis risk. Causal Analysis using Summary Effect Estimates (CAUSE) [22] was used as principal analytic device because it maximizes power (relative to other instrumental variable methods) by utilizing large sets of single nucleotide polymorphisms (SNPs) and its robustness to weak instrument bias and correlated horizontal pleiotropy [14, 22]. It uses genome-wide summary statistics to disentangle causality (i.e., SNPs are associated with periodontitis through their effect on physical activity) from correlated horizontal pleiotropy (i.e., SNPs are associated with physical activity and periodontitis through a shared heritable factor), while taking into account uncorrelated horizontal pleiotropy (i.e., SNPs are associated with physical activity through separate mechanisms). It uses Bayesian modelling to assess whether the sharing model (i.e., model that fixes the causal effect at zero) fits the data at least as well as the causal model (i.e., model that allows a causal effect different from zero).
For secondary analysis, we applied IVW analysis for weak, correlated instruments [23], and the robust adjusted profile score (RAPS) methodology [24], which enables the use of weak instruments, is robust to horizontal pleiotropy and considers potential measurement error in the exposure estimate. For IVW and RAPS, we used the clump_data function in the TwoSampleMR R package to select SNPs (P < 5 × 10−6) for each physical activity measure and performed linkage disequilibrium (LD) clumping (r2 < 0.2) using the European reference panel from the 1000 Genomes Project Phase 3 v5 (Supplementary Table 1). For SNPs not found in the outcome datasets, we used proxy SNP with a high LD (r2 > 0.9). Results are presented as odds ratio (OR) per 1-standard deviation increment in MVPA (MET-minutes/week) or AVEACC. One standard deviation of AVEACC in the UK Biobank Study is approximately 8 milli-gravities (or 0.08 m/s2) of acceleration in a mean 5-s window [15]. The OR estimates for VPA were scaled to a doubling in prevalence [25]. A Benjamini–Hochberg false discovery rate (FDR) < 5% was used to correct the results for multiple testing.
Instrumental variable estimation assumes instruments are (1) associated with physical activity (relevance assumption), (2) independent of confounders of the association between the instruments and periodontitis (exchangeability assumption), and (3) only associated with periodontitis through their effect on physical activity (exclusion restriction assumption) [14, 26]. To test for potential violations of the relevance assumption, we calculated F-statistics and the R2 for each measure of physical activity. Violations of the exchangeability and exclusion restriction assumption can occur when the instrument affects the outcome but through different pathways. Although it is not possible to prove that the exchangeability and exclusion restriction assumptions hold, sensitivity analysis can be used to uncover possible violations of these assumptions. One approach includes the assessment of potential violation of the exclusion restriction assumption through evaluation of the heterogeneity of the individual SNP estimates and through pleiotropy-robust approaches, such as CAUSE or RAPS, which provide unbiased estimates in the presence of horizontal pleiotropy [14]. We assessed heterogeneity using Cochran Q and IGX2 statistics. The MR Egger intercept test was used to test for directional pleiotropy [26]. We performed the analysis using R version 4.1.3 (R Foundation for Statistical Computing) using the cause, MendelianRandomization, mr.raps, and TwoSampleMR packages. The study was conducted based on relevant analysis and reporting guidelines [27, 28].
Results
In our main analysis, genetically instrumented physical activity was analyzed in relation to periodontitis using Causal Analysis using Summary Effect Estimates (CAUSE) [22]. We did not find evidence supporting effects of moderate-to-vigorous physical activity (MVPA), vigorous physical activity (VPA), average acceleration (AVEACC), or fraction of accelerations > 425 mg (ACC425) on the risk of periodontitis (Table 2). For example, the OR for MVPA and AVEACC were 1.07 (95% credible interval: 0.87; 1.34) and 1.00 (95% credible interval: 0.98; 1.02), respectively. The CAUSE estimates were supported IVW and RAPS analyses (Table 2).
The instruments for MVPA, VPA, AVEACC, or ACC425 explained 8.8%, 5.8%, 3.8%, and 1.5% of the phenotypic variance. The minimum F-statistic was 20.9, suggesting sufficient instrument strength and no violation of the relevance assumption (Supplementary Table 1). There was no heterogeneity in the IVW analyses (Supplementary Table 2). The intercepts from the MR Egger regression were centered around zero and provided no evidence for unbalanced pleiotropy (Supplementary Table 2).
Discussion
This instrumental variable study used genetic variants as instruments for self-reported and accelerometer-assessed physical activity to estimate effects on periodontitis risk. The findings do not suggest effects of physical activity on periodontitis. The study leveraged the CAUSE approach that maximizes statistical power and is robust to weak instrument bias and correlated horizontal pleiotropy. The CAUSE findings were endorsed in sensitivity analyses that aimed to further rule out that weak instrument bias or violations of the exchangeability or exclusion restriction instrumental variable assumptions are responsible for the null findings.
The instrumental variable approach using genetic variants as instruments has been successfully applied to shed light on the relationships of physical activity with various cancers [29, 30], cardiovascular diseases [31], psychiatric diseases [32], and neurological conditions [33]. In part, these instrumental variable studies refuted the widely held view that exercise medicine is a universal tool for disease prevention. One example is dementia, where a large number of observational cohort studies showed an inverse association of physical activity and disease risk. Subsequent long-term cohort and instrumental variable studies revealed that the observed inverse association between physical activity and dementia was subject to reverse causation due to a decline in physical activity in prodromal disease [33, 34].
All available observational studies, except one [35], on physical activity and periodontitis are cross-sectional reducing the potential to infer cause-effect relations because the cross-sectional design does not ensure that the exposure precedes the outcome. With cross-sectional data, it is difficult to rule out reverse causation. In such designs, periodontal disease features may make individuals become less physically active and induce a spurious inverse association [36]. Another potential source of bias of the existing observational studies is unobserved confounding. For example, in a recent cross-sectional study [10], the authors used multivariable logistic regression to adjust the relationship between self-reported physical activity and periodontitis for age, sex, race, poverty level, education, smoking, and diabetes. Yet, there might have been additional unadjusted confounders (e.g., periodontitis risk factors that co-occur with low physical activity such as alcohol consumption, oral hygiene, regular dental visits) or partially adjusted confounders (e.g., due to imperfect measurement of smoking or non-linear association with age) that might have introduced residual confounding.
The study has limitations. First, the study population comprised only individuals of European ancestry. Although restricting the study to ethnically homogeneous populations minimizes population-stratification bias, our results may not be generalizable to other populations with different genetic backgrounds. Second, genetic variants for ACC425 only explained 1.5% of the phenotypic variability, which may have reduced statistical power. In the future, identification of more instruments that explain more variance in accelerometer-assessed vigorous activity could strengthen inference. Third, the GWAS of physical activity consisted of UK Biobank participants aged 40 to 70 years. Previous twin studies [37] suggest that the genetic contribution to physical activity decreases with age. By estimating instrument-physical activity associations in a sample of middle-aged and older adults, we might have underestimated the denominator effect of the ratio estimator [33]. Thus, when the effect of the instrument on exposure changes over time, the ratio estimator could represent a biased estimate of the effect of physical activity on periodontitis.
In conclusion, the present study provides little evidence that physical activity would help to prevent periodontitis. Large cohort studies with repeated and valid measurement of physical activity and periodontitis and large GWAS data to provide strong instruments for physical activity in instrumental variable studies are needed to further triangulate the available evidence [12].
Data availability
All data analyzed for this study are publicly available. The UK Biobank physical activity data can be accessed at https://klimentidis.lab.arizona.edu/content/data. Periodontitis summary data are available at https://data.bris.ac.uk/data/dataset/2j2rqgzedxlq02oqbb4vmycnc2.
References
Stöhr J, Barbaresko J, Neuenschwander M et al (2021) Bidirectional association between periodontal disease and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Sci Rep 11:13686. https://doi.org/10.1038/s41598-021-93062-6
Hajishengallis G (2000) (2022) Interconnection of periodontal disease and comorbidities: evidence, mechanisms, and implications. Periodontol 89:9–18. https://doi.org/10.1111/prd.12430
Powell KE, King AC, Buchner DM et al (2018) The Scientific Foundation for the Physical Activity Guidelines for Americans, 2nd Edition. J Phys Act Health 1–11. https://doi.org/10.1123/jpah.2018-0618
Bull FC, Al-Ansari SS, Biddle S et al (2020) World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 54:1451–1462. https://doi.org/10.1136/bjsports-2020-102955
Aune D, Norat T, Leitzmann M et al (2015) Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol 30:529–542. https://doi.org/10.1007/s10654-015-0056-z
Chow LS, Gerszten RE, Taylor JM et al (2022) Exerkines in health, resilience and disease. Nat Rev Endocrinol 18:273–289. https://doi.org/10.1038/s41574-022-00641-2
Sun L, Zhu J, Ling Y et al (2021) Physical activity and the risk of rheumatoid arthritis: evidence from meta-analysis and Mendelian randomization. Int J Epidemiol 50:1593–1603. https://doi.org/10.1093/ije/dyab052
Prins FM, Said MA, van de Vegte YJ et al (2019) Geneticallydetermined physical activity and its association with circulating blood cells. Genes (Basel) 10. https://doi.org/10.3390/genes10110908
Ferreira RdO, Corrêa MG, Magno MB et al (2019) Physical activity reduces the prevalence of periodontal disease: systematic review and meta-analysis. Front Physiol 10:234. https://doi.org/10.3389/fphys.2019.00234
Almohamad M, Krall Kaye E, Mofleh D et al (2022) The association of sedentary behaviour and physical activity with periodontal disease in NHANES 2011–2012. J Clin Periodontol 49:758–767. https://doi.org/10.1111/jcpe.13669
Imbens G, Rubin DB (2015) Causal inference for statistics, social and biomedical sciences: an introduction. Cambridge University Press, Cambridge
Munafò MR, Higgins JPT, Smith GD (2021) Triangulating evidence through the inclusion of genetically informed designs. Cold Spring Harb Perspect Med 11. https://doi.org/10.1101/cshperspect.a040659
Maciejewski ML, Brookhart MA (2019) Using instrumental variables to address bias from unobserved confounders. JAMA 321:2124–2125. https://doi.org/10.1001/jama.2019.5646
Sanderson E, Glymour MM, Holmes MV et al (2022) Mendelian randomization. Nat Rev Methods Primers 2. https://doi.org/10.1038/s43586-021-00092-5
Klimentidis YC, Raichlen DA, Bea J et al (2018) Genome-wide association study of habitual physical activity in over 377,000 UK Biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes (Lond) 42:1161–1176. https://doi.org/10.1038/s41366-018-0120-3
Fry A, Littlejohns TJ, Sudlow C et al (2017) Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 186:1026–1034. https://doi.org/10.1093/aje/kwx246
Guo W, Key TJ, Reeves GK (2019) Accelerometer compared with questionnaire measures of physical activity in relation to body size and composition: a large cross-sectional analysis of UK Biobank. BMJ Open 9:e024206. https://doi.org/10.1136/bmjopen-2018-024206
Doherty A, Jackson D, Hammerla N et al (2017) Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank Study. PLoS One 12:e0169649. https://doi.org/10.1371/journal.pone.0169649
Hildebrand M, van Hees VT, Hansen BH et al (2014) Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc 46:1816–1824. https://doi.org/10.1249/MSS.0000000000000289
Shungin D, Haworth S, Divaris K et al (2019) Genome-wide analysis of dental caries and periodontitis combining clinical and self-reported data. Nat Commun 10:2773. https://doi.org/10.1038/s41467-019-10630-1
Deng L, Zhang H, Yu K (2020) Power calculation for the general two-sample Mendelian randomization analysis. Genet Epidemiol 44:290–299. https://doi.org/10.1002/gepi.22284
Morrison J, Knoblauch N, Marcus JH et al (2020) Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat Genet 52:740–747. https://doi.org/10.1038/s41588-020-0631-4
Burgess S, Dudbridge F, Thompson SG (2016) Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med 35:1880–1906. https://doi.org/10.1002/sim.6835
Zhao Q, Wang J, Hemani G et al (2020) Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann Stat 48:1742–1769. https://doi.org/10.1214/19-AOS1866
Burgess S, Labrecque JA (2018) Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol 33:947–952. https://doi.org/10.1007/s10654-018-0424-6
Hemani G, Bowden J, Davey Smith G (2018) Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet 27:R195–R208. https://doi.org/10.1093/hmg/ddy163
Skrivankova VW, Richmond RC, Woolf BAR et al (2021) Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ 375:n2233. https://doi.org/10.1136/bmj.n2233
Burgess S, Davey Smith G, Davies NM et al (2020) Guidelines for performing Mendelian randomization investigations. Wellcome Open Res 4:186. https://doi.org/10.12688/wellcomeopenres.15555.2
Papadimitriou N, Dimou N, Tsilidis KK et al (2020) Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat Commun 11:597. https://doi.org/10.1038/s41467-020-14389-8
Baumeister S-E, Leitzmann MF, Bahls M et al (2020) Physical activity does not lower the risk of lung cancer. Cancer Res 80:3765–3769. https://doi.org/10.1158/0008-5472.CAN-20-1127
Bahls M, Leitzmann MF, Karch A et al (2021) Physical activity, sedentary behavior and risk of coronary artery disease, myocardial infarction and ischemic stroke: a two-sample Mendelian randomization study. Clin Res Cardiol 110:1564–1573. https://doi.org/10.1007/s00392-021-01846-7
Papiol S, Schmitt A, Maurus I et al (2021) Association between physical activity and schizophrenia: results of a 2-sample Mendelian randomization analysis. JAMA Psychiat 78:441–444. https://doi.org/10.1001/jamapsychiatry.2020.3946
Baumeister S-E, Karch A, Bahls M et al (2020) Physical activity and risk of Alzheimer disease: a 2-sample Mendelian randomization study. Neurology 95:e1897–e1905. https://doi.org/10.1212/WNL.0000000000010013
Kivimäki M, Singh-Manoux A, Pentti J et al (2019) Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. BMJ 365:l1495. https://doi.org/10.1136/bmj.l1495
Merchant AT, Pitiphat W, Rimm EB et al (2003) Increased physical activity decreases periodontitis risk in men. Eur J Epidemiol 18:891–898. https://doi.org/10.1023/a:1025622815579
VanderWeele TJ (2021) Can sophisticated study designs with regression analyses of observational data provide causal inferences? JAMA Psychiat 78:244–246. https://doi.org/10.1001/jamapsychiatry.2020.2588
Vink JM, Boomsma DI, Medland SE et al (2011) Variance components models for physical activity with age as modifier: a comparative twin study in seven countries. Twin Res Hum Genet 14:25–34. https://doi.org/10.1375/twin.14.1.25
Acknowledgements
The authors acknowledge and thank the investigators of the original GWAS for sharing summary data used in this study.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
SEB, SLR, MN, and HB contributed to conception, design, data acquisition, and interpretation, performed all statistical analyses, and drafted and critically revised the manuscript.
BE and MN contributed to conception and design and critically revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from UK Biobank study participants, and ethics approval of UK Biobank was given by the North West Multicentre Research Ethics Committee, the National Information Governance Board for Health & Social Care, and the Community Health Index Advisory Group. The individual studies participating in GLIDE had previously obtained relevant ethical approval and written participant consent. This study complied with all relevant ethical regulations, including the Declaration of Helsinki, and ethical approval for data collection and analysis was obtained by each study from local boards as described in the included GWAS.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baumeister, SE., Reckelkamm, S.L., Ehmke, B. et al. Physical activity and the risk of periodontitis: an instrumental variable study. Clin Oral Invest 27, 4803–4808 (2023). https://doi.org/10.1007/s00784-023-05109-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05109-9