Abstract
Objectives
The present study evaluated the effect of an enamel matrix derivative (EMD) and platelet-rich fibrin (PRF)-modified porcine-derived collagen matrix (PDCM) on human umbilical vein endothelial cells (HUVEC) in vitro.
Materials and methods
PDCM (mucoderm®) was prepared to 6 mm (±0.1 mm) diameter discs. PDCM samples were incubated with either EMD, PRF, or control solutions for 100 min at 4 °C before the experiments. Cell-inducing properties of test materials on HUVEC cells were tested with cell proliferation assays (MTT, PrestoBlue®), a cytotoxicity assay (ToxiLight®), a Boyden chamber migration assay, and a cell attachment assay. Scanning electron microscopy (SEM) imaging was performed to determine the surface and the architecture of the modified matrices.
Results
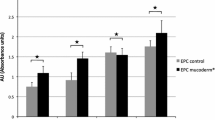
Cell proliferation was elevated in the EMD and PRF groups compared with control (p each ≤0.046). PRF modification increased HUVEC migration ability by 8-fold compared with both control and EMD groups (p each <0.001). Both treatments significantly promoted the cell attachment of HUVEC to PDCM, as assessed by direct cell counts on the matrices (p each <0.001).
Conclusions
HUVEC cell characteristics were overall improved by EMD- and PRF- modified PDCM. Adsorbed bioactive molecules to the PDCM surface may have contributed to a more preferable environment to surrounding cells.
Clinical relevance
The results may give evidence that PDCM modification with EMD or PRF, respectively, might be a useful approach to improve clinical outcomes, to prevent inflammatory reactions and wound-healing disturbances, and to expand the clinical application area of PDCM.




Similar content being viewed by others
References
Pabst AM, Happe A, Callaway A, Ziebart T, Stratul SI, Ackermann M, Konerding MA, Willershausen B, Kasaj A (2014) In vitro and in vivo characterization of porcine acellular dermal matrix for gingival augmentation procedures. J Periodontal Res 49(3):371–381. doi:10.1111/jre.12115
Atieh MA, Alsabeeha N, Tawse-Smith A, Payne AG (2015) Xenogeneic collagen matrix for periodontal plastic surgery procedures: a systematic review and meta-analysis. J Periodontal Res. doi:10.1111/jre.12333
Kim Y, Ko H, Kwon IK, Shin K (2016) Extracellular matrix revisited: roles in tissue engineering. Int Neurourol J 20(Suppl 1):S23–S29. doi:10.5213/inj.1632600.318
Hammarstrom L (1997) Enamel matrix, cementum development and regeneration. J Clin Periodontol 24(9 Pt 2):658–668
Gestrelius S, Andersson C, Lidstrom D, Hammarstrom L, Somerman M (1997) In vitro studies on periodontal ligament cells and enamel matrix derivative. J Clin Periodontol 24(9 Pt 2):685–692
Miron RJ, Sculean A, Cochran DL, Froum S, Zucchelli G, Nemcovsky C, Donos N, Lyngstadaas SP, Deschner J, Dard M, Stavropoulos A, Zhang Y, Trombelli L, Kasaj A, Shirakata Y, Cortellini P, Tonetti M, Rasperini G, Jepsen S, Bosshardt DD (2016) 20 years of enamel matrix derivative: the past, the present and the future. J Clin Periodontol. doi:10.1111/jcpe.12546
Koop R, Merheb J, Quirynen M (2012) Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: a systematic review. J Periodontol 83(6):707–720. doi:10.1902/jop.2011.110266
McGuire MK, Scheyer ET, Nunn M (2012) Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue: comparison of clinical parameters at 10 years. J Periodontol 83(11):1353–1362. doi:10.1902/jop.2012.110373
Bosshardt DD (2008) Biological mediators and periodontal regeneration: a review of enamel matrix proteins at the cellular and molecular levels. J Clin Periodontol 35(8 Suppl):87–105. doi:10.1111/j.1600-051X.2008.01264.x
Miron RJ, Dard M, Weinreb M (2015) Enamel matrix derivative, inflammation and soft tissue wound healing. J Periodontal Res 50(5):555–569. doi:10.1111/jre.12245
Choukroun J, Adda F, Schoeffler C, Vervelle A (2001) Une opportunite′ en paro-implantologie: le PRF. Implantodontie 42:55–62 French
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(3):e45–e50. doi:10.1016/j.tripleo.2005.07.009
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(3):e37–e44. doi:10.1016/j.tripleo.2005.07.008
Schar MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D (2015) Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res 473(5):1635–1643. doi:10.1007/s11999-015-4192-2
Marenzi G, Riccitiello F, Tia M, di Lauro A, Sammartino G (2015) Influence of leukocyte- and platelet-rich fibrin (L-PRF) in the healing of simple postextraction sockets: a split-mouth study. Biomed Res Int 2015:369273. doi:10.1155/2015/369273
Femminella B, Iaconi MC, Di Tullio M, Romano L, Sinjari B, D'Arcangelo C, De Ninis P, Paolantonio M (2016) Clinical comparison of platelet-rich fibrin and a gelatin sponge in the management of palatal wounds after epithelialized free gingival graft harvest: a randomized clinical trial. J Periodontol 87(2):103–113. doi:10.1902/jop.2015.150198
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ (2016) Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig 20(9):2353–2360. doi:10.1007/s00784-016-1719-1
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. doi:10.1056/NEJM197111182852108
Germain S, Monnot C, Muller L, Eichmann A (2010) Hypoxia-driven angiogenesis: role of tip cells and extracellular matrix scaffolding. Curr Opin Hematol 17(3):245–251. doi:10.1097/MOH.0b013e32833865b9
Michaelis UR (2014) Mechanisms of endothelial cell migration. Cell Mol Life Sci 71(21):4131–4148. doi:10.1007/s00018-014-1678-0
Zaidel-Bar R, Cohen M, Addadi L, Geiger B (2004) Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans 32(Pt3):416–420. doi:10.1042/BST0320416
Giancotti FG (1997) Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol 9(5):691–700
Kasaj A, Meister J, Lehmann K, Stratul SI, Schlee M, Stein JM, Willershausen B, Schmidt M (2012) The influence of enamel matrix derivative on the angiogenic activity of primary endothelial cells. J Periodontal Res 47(4):479–487. doi:10.1111/j.1600-0765.2011.01456.x
Bertl K, An N, Bruckmann C, Dard M, Andrukhov O, Matejka M, Rausch-Fan X (2009) Effects of enamel matrix derivative on proliferation/viability, migration, and expression of angiogenic factor and adhesion molecules in endothelial cells in vitro. J Periodontol 80(10):1622–1630. doi:10.1902/jop.2009.090157
Gestrelius S, Andersson C, Johansson AC, Persson E, Brodin A, Rydhag L, Hammarstrom L (1997) Formulation of enamel matrix derivative for surface coating. Kinetics and cell colonization. J Clin Periodontol 24(9 Pt 2):678–684
Miron RJ, Bosshardt DD, Laugisch O, Dard M, Gemperli AC, Buser D, Gruber R, Sculean A (2013) In vitro evaluation of demineralized freeze-dried bone allograft in combination with enamel matrix derivative. J Periodontol 84(11):1646–1654. doi:10.1902/jop.2013.120574
Lyngstadaas SP, Wohlfahrt JC, Brookes SJ, Paine ML, Snead ML, Reseland JE (2009) Enamel matrix proteins; old molecules for new applications. Orthod Craniofac Res 12(3):243–253. doi:10.1111/j.1601-6343.2009.01459.x
Philippart P, Daubie V, Pochet R (2005) Sinus grafting using recombinant human tissue factor, platelet-rich plasma gel, autologous bone, and anorganic bovine bone mineral xenograft: histologic analysis and case reports. Int J Oral Maxillofac Implants 20(2):274–281
Mazor Z, Peleg M, Garg AK, Luboshitz J (2004) Platelet-rich plasma for bone graft enhancement in sinus floor augmentation with simultaneous implant placement: patient series study. Implant Dent 13(1):65–72
Thor A, Wannfors K, Sennerby L, Rasmusson L (2005) Reconstruction of the severely resorbed maxilla with autogenous bone, platelet-rich plasma, and implants: 1-year results of a controlled prospective 5-year study. Clin Implant Dent Relat Res 7(4):209–220
Yang D, Lu X, Hong Y, Xi T, Zhang D (2013) The molecular mechanism of mediation of adsorbed serum proteins to endothelial cells adhesion and growth on biomaterials. Biomaterials 34(23):5747–5758. doi:10.1016/j.biomaterials.2013.04.028
Okubo K, Kobayashi M, Takiguchi T, Takada T, Ohazama A, Okamatsu Y, Hasegawa K (2003) Participation of endogenous IGF-I and TGF-beta 1 with enamel matrix derivative-stimulated cell growth in human periodontal ligament cells. J Periodontal Res 38(1):1–9
Nikitovic D, Chalkiadaki G, Berdiaki A, Aggelidakis J, Katonis P, Karamanos NK, Tzanakakis GN (2011) Lumican regulates osteosarcoma cell adhesion by modulating TGFbeta2 activity. Int J Biochem Cell Biol 43(6):928–935. doi:10.1016/j.biocel.2011.03.008
Warstat K, Meckbach D, Weis-Klemm M, Hack A, Klein G, de Zwart P, Aicher WK (2010) TGF-beta enhances the integrin alpha2beta1-mediated attachment of mesenchymal stem cells to type I collagen. Stem Cells Dev 19(5):645–656. doi:10.1089/scd.2009.0208
Curtis A, Wilkinson C (1997) Topographical control of cells. Biomaterials 18(24):1573–1583
Ranganathan AT, Chandran CR (2014) Platelet-rich fibrin in the treatment of periodontal bone defects. J Contemp Dent Pract 15(3):372–375
Pankov R, Yamada KM (2002) Fibronectin at a glance. J Cell Sci 115(Pt 20):3861–3863
Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Muller R, Livne E, Eming SA, Hubbell JA (2011) Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med 3(100):100ra189. doi:10.1126/scitranslmed.3002614
Miron RJ, Bosshardt DD, Buser D, Zhang Y, Tugulu S, Gemperli A, Dard M, Caluseru OM, Chandad F, Sculean A (2015) Comparison of the capacity of enamel matrix derivative gel and enamel matrix derivative in liquid formulation to adsorb to bone grafting materials. J Periodontol 86(4):578–587. doi:10.1902/jop.2015.140538
Zhang Y, Jing D, Buser D, Sculean A, Chandad F, Miron RJ (2016) Bone grafting material in combination with Osteogain for bone repair: a rat histomorphometric study. Clin Oral Investig 20(3):589–595. doi:10.1007/s00784-015-1532-2
Miron RJ, Fujioka-Kobayashi M, Zhang Y, Sculean A, Pippenger B, Shirakata Y, Kandalam U, Hernandez M (2016) Osteogain(R) loaded onto an absorbable collagen sponge induces attachment and osteoblast differentiation of ST2 cells in vitro. Clin Oral Investig. doi:10.1007/s00784-016-2019-5
Acknowledgments
Special thanks to Kerstin Bahr for SEM imaging. This work contains substantial parts of the dissertation to DMD of Jung Soo Park. Free samples of the tested matrix were allocated free of charge from Botiss (Botiss Biomaterials, Berlin, Germany).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
No funding was received for this study. The work was supported by the Department of Operative Dentistry and Periodontology, University Medical Center, Augustusplatz 2, 55131 Mainz, Germany. Free samples of the tested matrix were allocated free of charge from Botiss (Botiss Biomaterials, Berlin, Germany).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Electronic supplementary material
Suppl. 1
Automatic cell counting using ImageJ software. The original RGB image (a) is inverted and converted to gray-scale (8 bits) (b). “Image-based Tool for Counting Nuclei (ITCN)” command is then used to count cells. Counted particles are identified as red marks on the result picture (c). Sixteenfold magnified view of the area marked on Fig. 1c (d). (GIF 292 kb)
Suppl. 2
Microscopic images of SYTO® 11-stained HUVEC cells on PDCM samples. Cells were seeded on control, EMD, and PRF solution-pretreated PDCM samples. After 24 h, cells on matrices were stained with SYTO® Green, observed with an inverted microscope. Images were taken under 25-fold magnification from four different areas of each sample. Whereas cells are sparsely distributed on control sample (a), EMD- and PRF-treated PDCMs demonstrate an enormous amount of cells attached on the surface (b, d). In the PRF group, there were wide variations concerning the density of attached cells on the samples (c, d). Cell distributions were variable even within the same sample (c). a Control group; b EMD group; c, d PRF group. (GIF 412 kb)
Rights and permissions
About this article
Cite this article
Park, J.S., Pabst, A.M., Ackermann, M. et al. Biofunctionalization of porcine-derived collagen matrix using enamel matrix derivative and platelet-rich fibrin: influence on mature endothelial cell characteristics in vitro. Clin Oral Invest 22, 909–917 (2018). https://doi.org/10.1007/s00784-017-2170-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2170-7