Abstract
Objectives
For deep carious lesions, less invasive carious tissue removal is recommended. The resulting residual carious lesions might benefit from remineralization by lining or restoration materials. We aimed to compare mineral gains in artificial residual lesions provided by calcium hydroxide and glass hybrid materials in combination with pulpal fluid simulation.
Methods
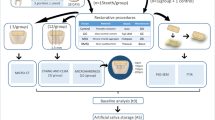
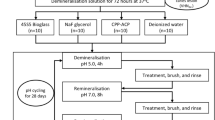
On the coronal aspect of human dentin discs (n = 20), artificial carious lesions were induced using acetic acid. Median mineral loss ΔZ [25th/75th percentiles] of resulting lesions was 1643 [1301/1858] vol% μm. One third of each disc served as baseline sample. The remaining disc was divided into four groups, each being covered with one experimental material (n = 20/group): flowable composite (control (CO)), setting or non-setting calcium hydroxide liner plus flowable composite (CH-S, CH-NS), and glass hybrid (GH). Samples were mounted in a dual-chamber device. Pulpal surfaces were exposed to simulated pulpal fluid at 2.94 kPa. Coronal surfaces were exposed to artificial saliva and rinsed with 200 ppm NaF every 2 weeks. After 12 weeks, mineral loss differences (ΔΔZ) were assessed using transverse microradiography. Electron probe microscopic analysis was used to measure fluoride and strontium concentrations.
Results
Mineral gains were not significantly different between CO (ΔΔZ = 372 [115/501] vol% μm), CH-S (ΔΔZ = 317 [229/919] vol% μm), or CH-NS (ΔΔZ = 292 [130/579] vol% μm; p > 0.05/Wilcoxon test) but significantly increased in GH (ΔΔZ = 1044 [751/1264] vol% μm, p < 0.001). Samples in GH showed fluoride and strontium enrichment deep into the dentin. Such enrichment was not found in CO.
Conclusions
Within the limitations of this study, GH, but not calcium hydroxide, provided coronal remineralization of residual carious lesions.
Clinical relevance
Glass hybrids might provide additional remineralization of residual carious lesions. The functional implications of this mineral gain need to be evaluated.



Similar content being viewed by others
References
Maltz M, Garcia R, Jardim JJ, de Paula LM, Yamaguti PM, Moura MS, Garcia F, Nascimento C, Oliveira A, Mestrinho HD (2012) Randomized trial of partial vs. stepwise caries removal. J Dent Res 91(11):1026–1031. doi:10.1177/0022034512460403
Mertz-Fairhurst EJ, Curtis JW, Ergle JW, Rueggeberg FA, Adair SM (1998) Ultraconservative and cariostatic sealed restorations: results at year 10. J Am Dent Assoc 129(1):55–66
Frencken J, Songpaisan Y, Phantumvanit P, Pilot T (1994) Atraumatic restorative treatment (ART) technique: evaluation after one year. Int Dent J 44:460–464
Bjørndal L (1999) Treatment of deep carious lesions with stepwise excavation. Pract Based Study Tandlaegebladet 103:498–506
Ricketts D, Lamont T, Innes NP, Kidd E, Clarkson JE (2013) Operative caries management in adults and children. Cochrane Database Syst Rev 28(3):CD003808
Griffin SO, Oong E, Kohn W, Vidakovic B, Gooch BF, Bader J, Clarkson J, Fontana MR, Meyer DM, Rozier RG, Weintraub JA, Zero DT (2008) The effectiveness of sealants in managing caries lesions. J Dent Res 87(2):169–174. doi:10.1177/154405910808700211
Oong EM, Griffin SO, Kohn WG, Gooch BF, Caufield PW (2008) The effect of dental sealants on bacteria levels in caries lesions. J Am Dent Assoc 139(3):271–278
Schwendicke F, Eggers K, Meyer-Lueckel H, Dorfer C, Kovalev A, Gorb S, Paris S (2015) In vitro induction of residual caries lesions in dentin: comparative mineral loss and nano-hardness analysis. Caries Res 49(3):259–265. doi:10.1159/000371897
Fusayama T, Kurosaki N (1972) Structure and removal of carious dentin. Int Dent J 22(3):401–411
Ito S, Saito T, Tay FR, Carvalho RM, Yoshiyama M, Pashley DH (2005) Water content and apparent stiffness of non-caries versus caries-affected human dentin. J Biomed Mater Res B Appl Biomater 72B(1):109–116. doi:10.1002/jbm.b.30130
Balooch M, Wu-Magidi IC, Balazs A, Lundkvist AS, Marshall SJ, Marshall GW, Siekhaus WJ, Kinney JH (1998) Viscoelastic properties of demineralized human dentin measured in water with atomic force microscope (AFM)-based indentation. J Biomed Mater Res 40(4):539–544
Kinney JH, Habelitz S, Marshall SJ, Marshall GW (2003) The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J Dent Res 82(12):957–961. doi:10.1177/154405910308201204
Kinney JH, Marshall SJ, Marshall GW (2003) The mechanical properties of human dentin: a critical review and re-evaluation of the dental literature. Crit Rev Oral Biol Med 14(1):13–29
Sauro S, Osorio R, Watson TF, Toledano M (2015) Influence of phosphoproteins’ biomimetic analogs on remineralization of mineral-depleted resin–dentin interfaces created with ion-releasing resin-based systems. Dent Mater 31(7):759–777. doi:10.1016/j.dental.2015.03.013
Deyhle H, Bunk O, Müller B (2011) Nanostructure of healthy and caries-affected human teeth. Nanomed Nanotechnol Biol Med 7(6):694–701. doi:10.1016/j.nano.2011.09.005
Klont B, ten Cate JM (1991) Remineralization of bovine incisor root lesions in vitro: the role of the collagenous matrix. Caries Res 25(1):39–45
Koutsoukos PG, Nancollas GH (1981) Crystal growth of calcium phosphates—epitaxial considerations. J Cryst Growth 53(1):10–19. doi:10.1016/0022-0248(81)90051-8
Colfen H (2010) Biomineralization: a crystal-clear view. Nat Mater 9(12):960–961. doi:10.1038/nmat2911
Miyauchi H, Iwaku M, Fusayama T (1978) Physiological recalcification of carious dentin. Bull Tokyo Med Dent Univ 25(3):169–179
Corralo DJ, Maltz M (2013) Clinical and ultrastructural effects of different liners/restorative materials on deep carious dentin: a randomized clinical trial. Caries Res 47(3):243–250
Marchi JJ, Froner AM, Alves HL, Bergmann CP, Araujo FB (2008) Analysis of primary tooth dentin after indirect pulp capping. J Dent Child (Chicago, Ill) 75(3):295–300
Conrado CA (2004) Remineralization of carious dentin. I: in vitro microradiographic study in human teeth capped with calcium hydroxide. Braz Dent J 15:59–62
Conrado CA (2004) Remineralization of carious dentin. II: in vivo microradiographic and chemical studies in human permanent teeth capped with calcium hydroxide. Braz Dent J 15(3):186–189
Eidelman E, Finn SB, Koulourides T (1965) Remineralization of carious dentin treated with calcium hydroxide. J Dent Child 32(4):218–225
Cox CF, Hafez AA, Akimoto N, Otsuki M, Mills JC (1999) Biological basis for clinical success: pulp protection and the tooth-restoration interface. Pract Periodontics Aesthet Dent PPAD 11(7):819–826 quiz 827
Oen KT, Thompson VP, Vena D, Caufield PW, Curro F, Dasanayake A, Ship JA, Lindblad A (2007) Attitudes and expectations of treating deep caries: a PEARL Network survey. Gen Dent 55(3):197–203
Schwendicke F, Meyer-Lueckel H, Dorfer C, Paris S (2013) Attitudes and behaviour regarding deep dentin caries removal: a survey among German dentists. Caries Res 47(6):566–573. doi:10.1159/000351662
Sauro S, Pashley DH, Montanari M, Chersoni S, Carvalho RM, Toledano M, Osorio R, Tay FR, Prati C (2007) Effect of simulated pulpal pressure on dentin permeability and adhesion of self-etch adhesives. Dental Materials 23(6):705–713. doi:10.1016/j.dental.2006.06.010
Sauro S, Thompson I, Watson TF (2011) Effects of common dental materials used in preventive or operative dentistry on dentin permeability and remineralization. Oper Dent 36(2):222–230. doi:10.2341/10-225-l
Buskes JA, Christoffersen J, Arends J (1985) Lesion formation and lesion remineralization in enamel under constant composition conditions. A new technique with applications. Caries Res 19(6):490–496
Wang Z, Jiang T, Sauro S, Wang Y, Xing W, Liang S, Sa Y, Zhang C, Shen Y, Haapasalo M (2012) Nerve-targeted desensitizing toothpastes occlude dentin tubules and induce mineral precipitation. Am J Dent 25(1):26–30
Schwendicke F, Al-Abdi A, Meyer-Lückel H, Paris S (2015) Pulpal remineralisation of artificial residual caries lesions in vitro. Caries Res 49(6):591–594
Wong L, Sissons CH (2001) A comparison of human dental plaque microcosm biofilms grown in an undefined medium and a chemically defined artificial saliva. Arch Oral Biol 46(6):477–486. doi:10.1016/s0003-9969(01)00016-4
Shellis RP (1994) Effects of a supersaturated pulpal fluid on the formation of caries-like lesions on the roots of human teeth. Caries Res 28(1):14–20
Ciucchi B, Bouillaguet S, Holz J, Pashley D (1995) Dentinal fluid dynamics in human teeth, in vivo. J Endod 21(4):191–194. doi:10.1016/s0099-2399(06)80564-9
Alves LS, Fontanella V, Damo AC, Ferreira de Oliveira E, Maltz M (2010) Qualitative and quantitative radiographic assessment of sealed carious dentin: a 10-year prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 109(1):135–141. doi:10.1016/j.tripleo.2009.08.021
Chen Z, Cao S, Wang H, Li Y, Kishen A, Deng X, Yang X, Wang Y, Cong C, Wang H, Zhang X (2015) Biomimetic remineralization of demineralized dentine using scaffold of CMC/ACP nanocomplexes in an in vitro tooth model of deep caries. PLoS One 10(1):e0116553. doi:10.1371/journal.pone.0116553
Schwendicke F, Al-Abdi A, Meyer-Lückel H, Paris S (2015) Pulpal remineralisation of artificial residual caries lesions in vitro. Caries Res Revision 49:591–594. doi:10.1159/000440906
Schwendicke F, Tu YK, Hsu LY, Gostemeyer G (2015) Antibacterial effects of cavity lining: a systematic review and network meta-analysis. J Dent. doi:10.1016/j.jdent.2015.07.001
Marchi JJ, de Araujo FB, Fröner AM, Straffon LH, Nör J (2006) Indirect pulp capping in the primary dentition: a 4 year follow-up study. J Clin Pediatr Dent 31(2):68–71
Dalpian DM, Casagrande L, Franzon R, Dutra GM, de Araujo FB (2012) Dentin microhardness of primary teeth undergoing partial carious removal. J Clin Pediatr Dent 36(4):363–367
Schwendicke F, Goestemeyer G, Gluud C (2015) Cavity lining after excavating caries lesions: meta-analysis and trial sequential analysis of randomized clinical trials. J Dent. doi:10.1016/j.jdent.2015.07.017
Ardeshna SM, Qualtrough AJ, Worthington HV (2002) An in vitro comparison of pH changes in root dentine following canal dressing with calcium hydroxide points and a conventional calcium hydroxide paste. Int Endod J 35(3):239–244
Kitasako Y, Nakajima M, Foxton RM, Aoki K, Pereira PN, Tagami J (2003) Physiological remineralization of artificially demineralized dentin beneath glass ionomer cements with and without bacterial contamination in vivo. Oper Dent 28(3):274–280
Melo MA, Morais WA, Passos VF, Lima JP, Rodrigues LK (2014) Fluoride releasing and enamel demineralization around orthodontic brackets by fluoride-releasing composite containing nanoparticles. Clin Oral Invest 18(4):1343–1350. doi:10.1007/s00784-013-1073-5
Knight GM, McIntyre JM, Craig GG, Mulyani (2006) Ion uptake into demineralized dentine from glass ionomer cement following pretreatment with silver fluoride and potassium iodide. Aust Dent J 51(3):237–241
Knight GM, McIntyre JM, Craig GG, Mulyani ZPS, Gully NJ (2007) An in vitro investigation of marginal dentine caries abutting composite resin and glass ionomer cement restorations. Aust Dent J 52(3):187–192
Creanor SL, Awawdeh LA, Saunders WP, Foye RH, Gilmour WH (1998) The effect of a resin-modified glass ionomer restorative material on artificially demineralised dentine caries in vitro. J Dent 26(5–6):527–531
Yang B, Flaim G, Dickens SH (2011) Remineralization of human natural caries and artificial caries-like lesions with an experimental whisker-reinforced ART composite. Acta Biomater 7(5):2303–2309. doi:10.1016/j.actbio.2011.01.002
Ngo HC, Mount G, Mc Intyre J, Tuisuva J, Von Doussa RJ (2006) Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: an in vivo study. J Dent 34(8):608–613. doi:10.1016/j.jdent.2005.12.012
Mukai M, Ikeda M, Yanagihara T, Hara G, Kato K, Nakagaki H, Robinson C (1993) Fluoride uptake in human dentine from glass-ionomer cement in vivo. Arch Oral Biol 38(12):1093–1098
Smales RJ, Ngo HC, Yip KH, Yu C (2005) Clinical effects of glass ionomer restorations on residual carious dentin in primary molars. Am J Dent 18(3):188–193
Peters MC, Bresciani E, Barata TJ, Fagundes TC, Navarro RL, Navarro MF, Dickens SH (2010) In vivo dentin remineralization by calcium-phosphate cement. J Dent Res 89(3):286–291. doi:10.1177/0022034509360155
Kuhn E, Chibinski AC, Reis A, Wambier DS (2014) The role of glass ionomer cement on the remineralization of infected dentin: an in vivo study. Pediatr Dent 36(4):118–124
Kim YK, Yiu CK, Kim JR, Gu L, Kim SK, Weller RN, Pashley DH, Tay FR (2010) Failure of a glass ionomer to remineralize apatite-depleted dentin. J Dent Res 89(3):230–235. doi:10.1177/0022034509357172
Xie D, Zhao J, Weng Y, Park JG, Jiang H, Platt JA (2008) Bioactive glass-ionomer cement with potential therapeutic function to dentin capping mineralization. Eur J Oral Sci 116(5):479–487. doi:10.1111/j.1600-0722.2008.00562.x
Arends J, Christoffersen J, Ruben J, Jongebloed WL (1989) Remineralization of bovine dentine in vitro. The influence of the F content in solution on mineral distribution. Caries Res 23(5):309–314
Hotta M, Li Y, Sekine I (2001) Mineralization in bovine dentin adjacent to glass-ionomer restorations. J Dent 29(3):211–215
ten Cate JM, van Duinen RN (1995) Hypermineralization of dentinal lesions adjacent to glass-ionomer cement restorations. J Dent Res 74(6):1266–1271
Wilson AD, Prosser HJ, Powis DM (1983) Mechanism of adhesion of polyelectrolyte cements to hydroxyapatite. J Dent Res 62(5):590–592
Bertassoni LE, Habelitz S, Kinney JH, Marshall SJ, Marshall GW Jr.(2009) Biomechanical perspective on the remineralization of dentin. Caries Res 43(1):70–77. doi:10.1159/000201593
Niu LN, Zhang W, Pashley DH, Breschi L, Mao J, Chen JH, Tay FR (2014) Biomimetic remineralization of dentin. Dent Mater Off Publ Acad Dent Mater 30(1):77–96. doi:10.1016/j.dental.2013.07.013
Bresciani E, Wagner WC, Navarro MF, Dickens SH, Peters MC (2010) In vivo dentin microhardness beneath a calcium-phosphate cement. J Dent Res 89(8):836–841. doi:10.1177/0022034510369292
Attar N, Onen A (2002) Fluoride release and uptake characteristics of aesthetic restorative materials. J Oral Rehabil 29(8):791–798
Attard N, Barzilay I (2003) A modified impression technique for accurate registration of peri-implant soft tissues. J (Can Dent Assoc) 69(2):80–83
Cildir SK, Sandalli N (2005) Fluoride release/uptake of glass-ionomer cements and polyacid-modified composite resins. Dent Mater J 24(1):92–97
Acknowledgments
We thank Dr. Jörg Nissen, Technical University Berlin, for EPMA analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Extracted human permanent molars were obtained with informed consent based on an ethics approved protocol (EA4/102/14).
Conflict of interest
Experimental determination of remineralization effects by GH was funded by GC Europe, Leuven, Belgium. The funders had no role in design, conduct, evaluation or interpretation of the study, or writing the manuscript.
Funding
FS receives a grant from the German Research Foundation (SCHW 1766/2-1). This study was co-funded by GC Europe, Leuven, Belgium. The funders had no role in design, conduct, evaluation, or interpretation of the study or writing the manuscript.
Informed consent
Extracted human permanent molars were obtained with informed consent based on an ethics approved protocol (EA4/102/14).
Rights and permissions
About this article
Cite this article
Al-Abdi, A., Paris, S. & Schwendicke, F. Glass hybrid, but not calcium hydroxide, remineralized artificial residual caries lesions in vitro . Clin Oral Invest 21, 389–396 (2017). https://doi.org/10.1007/s00784-016-1803-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1803-6