Abstract
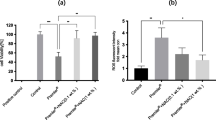
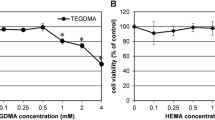
Dental composites are a source of residual monomers that are released into the oral environment. Since monomers act on cultured cells through reactive oxygen species (ROS), we hypothesized that composites generate ROS associated with cytotoxicity. Human pulp-derived cells were exposed to extracts of methacrylate-based materials including triethylene glycol dimethacrylate and 2-hydroxyethyl methacrylate-free composites (Tetric Ceram, Tetric EvoCeram, els, els flow, Solitaire 2) and a silorane-based composite (Hermes III). The materials were polymerized in the presence and absence of a polyester film and then extracted in culture medium. The generation of ROS was measured by flow cytometry, and cytotoxicity was determined as well. Methacrylate-based composites reduced cell survival but varied in efficiency. Undiluted extracts of Solitaire 2 specimens prepared in the absence of a polyester film reduced cell survival to 26% compared with untreated cultures. Cytotoxicity was reduced when specimens were covered with a polyester film during preparation. Cytotoxicity of the composites was ranked as follows: Solitaire 2 >> els flow > Tetric Ceram = Tetric EvoCeram = els > Hermes III. The generation of ROS followed the same pattern as detected with cytotoxic effects. A positive correlation was found between ROS production and cell survival caused by extracts made from materials not covered with a polyester film. These findings suggest that components released from composites affect cellular signaling networks through ROS formation. Regenerative and reparative capacities of the dentine–pulp complex may be impaired by biologically active resin monomers released from composite restorations.





Similar content being viewed by others
References
Sloan AJ, Smith AJ (2007) Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis 13:151–157
Smith AJ, Cassidy N, Perry H, Begue-Kirn C, Ruch JV, Lesot H (1995) Reactionary dentinogenesis. Int J Dev Biol 39:273–280
Ferracane JL, Cooper PR, Smith AJ (2010) Can interaction of materials with the dentin–pulp complex contribute to dentin regeneration? Odontology 98:2–14
Seiss M, Langer C, Hickel R, Reichl FX (2009) Quantitative determination of TEGDMA, BHT, and DMABEE in eluates from polymerized resin-based dental restorative materials by use of GC/MS. Arch Toxicol 83:1109–1115
Shawkat ES, Shortall AC, Addison O, Palin WM (2009) Oxygen inhibition and incremental layer bond strengths of resin composites. Dent Mater 25:1338–1346
Rueggeberg FA, Margeson DH (1990) The effect of oxygen inhibition on an unfilled/filled composite system. J Dent Res 69:1652–1658
Noda M, Wataha JC, Kaga M, Lockwood PE, Volkmann KR, Sano H (2002) Components of dentinal adhesives modulate heat shock protein 72 expression in heat-stressed THP-1 human monocytes at sublethal concentrations. J Dent Res 81:265–269
Michelsen VB, Moe G, Skalevik R, Jensen E, Lygre H (2007) Quantification of organic eluates from polymerized resin-based dental restorative materials by use of GC/MS. J Chromatogr B Analyt Technol Biomed Life Sci 850:83–91
Schweikl H, Hiller KA, Eckhardt A, Bolay C, Spagnuolo G, Stempfl T, Schmalz G (2008) Differential gene expression involved in oxidative stress response caused by triethylene glycol dimethacrylate. Biomaterials 29:1377–1387
Spagnuolo G, D'Anto V, Cosentino C, Schmalz G, Schweikl H, Rengo S (2006) Effect of N-acetyl-L-cysteine on ROS production and cell death caused by HEMA in human primary gingival fibroblasts. Biomaterials 27:1803–1809
Engelmann J, Volk J, Leyhausen G, Geurtsen W (2005) ROS formation and glutathione levels in human oral fibroblasts exposed to TEGDMA and camphorquinone. J Biomed Mater Res B Appl Biomater 75:272–276
Matsuzawa A, Ichijo H (2005) Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signal 7:472–481
Lefeuvre M, Bourd K, Loriot MA, Goldberg M, Beaune P, Perianin A, Stanislawski L (2004) TEGDMA modulates glutathione transferase P1 activity in gingival fibroblasts. J Dent Res 83:914–919
Schweikl H, Hartmann A, Hiller KA, Spagnuolo G, Bolay C, Brockhoff G, Schmalz G (2007) Inhibition of TEGDMA and HEMA-induced genotoxicity and cell cycle arrest by N-acetylcysteine. Dent Mater 23:688–695
Smith AJ (2002) Pulpal responses to caries and dental repair. Caries Res 36:223–232
Spagnuolo G, D'Anto V, Valletta R, Strisciuglio C, Schmalz G, Schweikl H, Rengo S (2008) Effect of 2-hydroxyethyl methacrylate on human pulp cell survival pathways ERK and AKT. J Endod 34:684–688
Galler KM, Schweikl H, Hiller KA, Cavender AC, Bolay C, D’Souza RN, Schmalz G. TEGDMA reduces mineralization in dental pulp cells. J Dent Res, in press
Weinmann W, Thalacker C, Guggenberger R (2005) Siloranes in dental composites. Dent Mater 21:68–74
ISO 10993-12:2007. Biological evaluation of medical devices -- Part 12: Sample preparation and reference materials. International Organisation for Standardization, Case postale 56, CH-1211 Geneva 20, Switzerland
Myhre O, Andersen JM, Aarnes H, Fonnum F (2003) Evaluation of the probes 2', 7'-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 65:1575–1582
Galler K, Schweikl H, Thonemann B, Schmalz G (2006) Human pulp-derived cells immortalized with SV40. Eur J Oral Sci 114:138–146
Gilles RJ, Didier N, Denton M (1986) Determination of cell number in monolayer cultures. Anal Biochem 159:109–113
Forman HJ, Fukuto JM, Torres M (2004) Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287:C246–C256
Stanislawski L, Lefeuvre M, Bourd K, Soheili-Majd E, Goldberg M, Perianin A (2003) TEGDMA-induced toxicity in human fibroblasts is associated with early and drastic glutathione depletion with subsequent production of oxygen reactive species. J Biomed Mater Res A 66:476–482
Engelmann J, Janke V, Volk J, Leyhausen G, von NN, Schlegelberger B, Geurtsen W (2004) Effects of BisGMA on glutathione metabolism and apoptosis in human gingival fibroblasts in vitro. Biomaterials 25:4573–4580
Nocca G, D'Antò V, Desiderio C, Rossetti DV, Valletta R, Baquala AM, Schweikl H, Lupi A, Rengo S, Spagnuolo G (2010) N-acetyl cysteine directed detoxification of 2-hydroxyethyl methacrylate by adduct formation. Biomaterials 31:2508–2516
Paranjpe A, Cacalano NA, Hume WR, Jewett A (2009) N-acetyl cysteine mediates protection from 2-hydroxyethyl methacrylate induced apoptosis via nuclear factor kappa B-dependent and independent pathways: potential involvement of JNK. Toxicol Sci 108:356–366
Krifka S, Petzel C, Hiller KA, Frank EM, Bosl C, Spagnuolo G, Reichl FX, Schmalz G, Schweikl H (2010) Resin monomer-induced differential activation of MAP kinases and apoptosis in mouse macrophages and human pulp cells. Biomaterials 31:2964–2975
LeBel CP, Ishiropoulos H, Bondy SC (1992) Evaluation of the probe 2, 7-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Demirci M, Hiller KA, Bosl C, Galler K, Schmalz G, Schweikl H (2008) The induction of oxidative stress, cytotoxicity, and genotoxicity by dental adhesives. Dent Mater 24:362–371
Spagnuolo G, Galler K, Schmalz G, Cosentino C, Rengo S, Schweikl H (2004) Inhibition of phosphatidylinositol 3-kinase amplifies TEGDMA-induced apoptosis in primary human pulp cells. J Dent Res 83:703–707
Schweikl H, Spagnuolo G, Schmalz G (2006) Genetic and cellular toxicology of dental resin monomers. J Dent Res 85:870–877
Schweikl H, Hiller KA, Bolay C, Kreissl M, Kreismann W, Nusser A, Steinhauser S, Wieczorek J, Vasold R, Schmalz G (2005) Cytotoxic and mutagenic effects of dental composite materials. Biomaterials 26:1713–1719
Michelsen VB, Moe G, Strøm MB, Jensen E, Lygre H (2008) Quantitative analysis of TEGDMA and HEMA eluted into saliva from two dental composites by use of GC/MS and tailor-made internal standards. Dent Mater 24:724–731
Wataha JC, Lockwood PE, Bouillaguet S, Noda M (2003) In vitro biological response to core and flowable dental restorative materials. Dent Mater 19:25–31
Al-Hiyasat AS, Darmani H, Milhem MM (2005) Cytotoxicity evaluation of dental resin composites and their flowable derivatives. Clin Oral Investig 9:21–25
Kopperud HM, Schmidt M, Kleven IS (2010) Elution of substances from a silorane-based dental composite. Eur J Oral Sci 118:100–102
Schweikl H, Schmalz G, Weinmann W (2004) The induction of gene mutations and micronuclei by oxiranes and siloranes in mammalian cells in vitro. J Dent Res 83:17–21
Schweikl H, Schmalz G, Weinmann W (2002) Mutagenic activity of structurally related oxiranes and siloranes in Salmonella typhimurium. Mutat Res 521:19–27
Brackett MG, Bouillaguet S, Lockwood PE, Rotenberg S, Lewis JB, Messer RL, Wataha JC (2007) In vitro cytotoxicity of dental composites based on new and traditional polymerization chemistries. J Biomed Mater Res B Appl Biomater 81:397–402
Acknowledgments
This study was supported by the University of Regensburg Medical Center. The authors are grateful to Mrs. Christine Ross-Cavanna for a critical reading of the manuscript.
Conflict of interest
The authors do not have any conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krifka, S., Seidenader, C., Hiller, KA. et al. Oxidative stress and cytotoxicity generated by dental composites in human pulp cells. Clin Oral Invest 16, 215–224 (2012). https://doi.org/10.1007/s00784-010-0508-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-010-0508-5