Abstract
A series of Ni(II) sandwich-like coordinated compounds were synthesized by the reaction of nickel dichloride and ten 4′-(4-substituent phenyl)-2′,2′:6′,2″-terpyridine ligands, and their structures were confirmed by elemental analysis, FT-IR, ESI–MS, solid state ultraviolet spectroscopy and X-ray single crystal diffraction analysis. Three human cancer cell lines and a normal human cell line were used for anti-proliferation potential study: human lung cancer cell line (A549), human esophageal cancer cell line (Eca-109), human liver cancer cells (Bel-7402) and normal human liver cells (HL-7702). The results show that these nickel complexes possess good inhibitory effects on the cancer cells, outperforming the commonly used clinical chemotherapy drug cisplatin. Especially, complexes 3 (-methoxyl) and 7 (-fluoro) have strong inhibitory ability against Eca-109 cell line with IC50 values of 0.223 μM and 0.335 μM, complexes 4 and 6 showed certain cell selectivity, and complex 6 can inhibit cancer cells and slightly poison normal cells when the concentration was controlled. The ability of these complexes binding to CT-DNA was studied by UV titration and CD spectroscopy, and CD spectroscopy was also used to study the secondary structural change of BSA under the action of the complexes. The binding of these complexes with DNA, DNA-Topo I and bovine serum protein has been simulated by molecular docking software, and the docking results and optimal binding conformation data showed that they interacted with DNA in the mode of embedded binding, which is consistent with the experimental results. These complexes are more inclined to move to the cleavage site when docking with DNA-Topo I, so as to play a role of enzyme cleavage, while BSA promotes the action of the complexes by binding to effective binding sites.
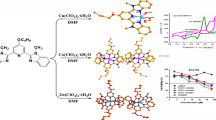
Graphical abstract











Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE et al (2021) Cancer statistics, 2021. CA Cancer J Clin 71:7–33
Bray F, Ferlay J, Soerjomataram I et al (2020) Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 70(4):394–424
Harmers FP, Gispen WH, Neijt JP (1991) Neurotoxic side-effects of cisplatin. Eur J Cancer (1990) 27(3):372–376
Huang W, Liu Y, Wang J et al (2018) Small-molecule compounds targeting the STAT3 DNA-binding domain suppress survival of cisplatin-resistant human ovarian cancer cells by inducing apoptosis. Eur J Med Chem 157:887–897
HeidarpoorSaremi L, DadashiNoshahr K, Ebrahimi A et al (2021) Multi-stage screening to predict the specific anticancer activity of Ni(II) mixed-ligand complex on gastric cancer cells; biological activity, FTIR spectrum, DNA binding behavior and simulation studies. Spectrochim Acta Part A Mol Biomol Spectrosc 251:1386–1425
El-Gammal OA, Mohamed FS, Rezk GN et al (2021) Synthesis, characterization, catalytic, DNA binding and antibacterial activities of Co(II), Ni(II) and Cu(II) complexes with new Schiff base ligand. J Mol Liq 326:115–223
Wasylenko DJ, Ganesamoorthy C, Henderson MA et al (2010) Electronic modification of the [Ru II (tpy)(bpy)(OH)2]2+ scaffold: effects on catalytic water oxidation. J Am Chem Soc 132(45):16094–16106
Concepcion JJ, Tsai M, Muckerman JT et al (2010) Mechanism of water oxidation by single-site ruthenium complex catalysts. J Am Chem Soc 132(5):1545–1557
Zheng M, Tan H, Xie Z et al (2013) Fast response and high sensitivity europium metal organic framework fluorescent probe with chelating terpyridine sites for Fe3+. ACS Appl Mater Interfaces 5(3):1078–1083
Baranoff E, Collin J, Flamigni L et al (2004) From ruthenium(II) to iridium(II): 15 years of triads based on bis-terpyridine complexes. Chem Soc Rev 33(3):147–155
Howe-Grant M, Wu KC, Bauer WR et al (1976) Binding of platinum and palladium metallointercalation reagents. Biochem Cell Biol 19(15):4336–4339
Eryazici I, Moorefield CN, Newkome GR (2008) Square-planar Pd(II), Pt(II), and Au(III) terpyridine complexes: their syntheses, physical properties, supramolecular constructs, and biomedical activities. Chem Rev 108(6):1834–1895
Ma Z, Lu W, Liang B et al (2013) Synthesis, characterization, photoluminescent and thermal properties of zinc(II) 4′-phenyl-terpyridine compounds. New J Chem 37(5):1529–1537
Lowe G, Droz AS, Vilaivan T et al (1999) Cytotoxicity of 2,2’:6,2’’-terpyridineplatinum(II) complexes against human ovarian carcinoma. J Med Chem 42(16):3167–3174
Liang X, Jiang J, Xue X et al (2019) Synthesis, characterization, photoluminescence, anti-tumor activity, DFT calculations and molecular docking with proteins of zinc(II) halogen substituted terpyridine compounds. Dalton Trans 48(28):10488–10504
Liu R, Yan H, Jiang J et al (2020) Synthesis, characterization, photoluminescence, molecular docking and bioactivity of Zinc(II) compounds based on different substituents. Molecules 25(15):3459–3479
Li J, Yan H, Wang Z et al (2021) Copper chloride complexes with substituted 4’-phenyl-terpyridine ligands: synthesis, characterization, antiproliferative activities and DNA interactions. Dalton Trans 50(23):8243–8257
Huang L, Liu R, Li J et al (2019) Synthesis, characterization, anti-tumor activity, photo-luminescence and BHb/HHb/Hsp90 molecular docking of Zinc(II) hydroxyl-terpyridine complexes. J Inorg Biochem 201:134–162
Li J, Liu R, Jiang J et al (2020) Synthesis, characterization, photoluminescence, antiproliferative activity, and DNA interaction of cadmium(II) substituted 4’-phenyl-terpyridine compounds. J Inorg Biochem 210:131–165
Li J, Liu R, Jiang J et al (2019) Zinc(II) terpyridine complexes: substituent effect on photoluminescence, antiproliferative activity, and DNA interaction. Molecules 24(24):4519–4546
Ma Z, Zhang B, Guedes Da Silva MFC et al (2020) Synthesis, characterization, thermal properties and antiproliferative potential of copper(ii) 4’-phenyl-terpyridine compounds. Dalton Trans 45(12):5339–5355
Wang S, Chu W, Wang Y et al (2013) Synthesis, characterization and cytotoxicity of Pt(II), Pd(II), Cu(II) and Zn(II) complexes with 4’-substituted terpyridine. Appl Organomet Chem 27:373–379
Wei C, Ren L, Gao N (2013) Interactions of terpyridines and their Pt(II) complexes with G-quadruplex DNAs and telomerase inhibition. Int J Biol Macromol 57:1–8
Kuehnel MF, Orchard KL, Dalle KE et al (2017) Selective photocatalytic CO2 reduction in water through anchoring of a molecular Ni catalyst on CdS nanocrystals. J Am Chem Soc 139:7217–7223
White F, Sykora RE (2014) Crystal structure of bis(2,2′:6′,2′′-terpyridine-k3 N, N′, N′′)nickel(II) dicyanidoaurate(I). Acta Crystallogr Sect E Struct Rep Online 70:519–521
Ciszewski JT, Mikhaylov DY, Holin KV et al (2011) Redox trends in terpyridine nickel complexes. Inorg Chem 50:8630–8635
Budnikova Y, Vicic D, Klein A (2018) Exploring mechanisms in Ni terpyridine catalyzed C-C cross-coupling reactions-a review. Inorganics 6(1):18–36
Constable EC, Lewis J, Schröder M (1982) The preparation and ekctrochemistry of complexes of 4’, 4-diphenyl-2,2’: 6’,2’’ WfwePyrkl. Polyhedron 3(1):311–312
Agilent (2012) CrysAlis PRO, Version. Agilent Technologies, Yarnton, UK 171:40–53
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem 1(71):3–8
Dolomanov OV, Bourhis L, Puschmann H et al (2008) OLEX2: a portable molecular graphicsOLEX2. Acta Crystallogr A Found Adv A64:C219
Lj F (1999) WinGX suite for small-molecule single-crystal crystallography. J Appl Crystallogr 4(32):837–838
Ma Z, Wei L, Alegria ECBA et al (2014) Synthesis and characterization of copper(II) 4’-phenyl-terpyridine compounds and catalytic application for aerobic oxidation of benzylic alcohols. Dalton Trans 43(10):4048–4058
Jiang J, Li J, Liu C et al (2020) Study on the substitution effects of zinc benzoate terpyridine complexes on photoluminescence, antiproliferative potential and DNA binding properties. J Biol Inorg Chem 25:311–324
Alsaeedi MS, Babgi BA, Hussien MA et al (2020) DNA-binding and anticancer activity of binuclear gold(I) alkynyl complexes with a phenanthrenyl bridging ligand. Molecules 25(5):1033–1047
HashemzadehEsfahani N, Salami F, Saberi Z et al (2019) DNA G-quadruplexes binding and antitumor activity of palladium aryl oxime ligand complexes encapsulated in either albumin or algal cellulose nanoparticles. Colloids Surf B 176:70–79
Kellett A, Molphy Z, Slator C et al (2019) Molecular methods for assessment of non-covalent metallodrug-DNA interactions. Chem Soc Rev 48(4):971–988
Sirajuddin M, Ali S, Badshah A (2013) Drug-DNA interactions and their study by UV–visible, fluorescence spectroscopies and cyclic voltametry. J Photochem Photobiol B 124:1–19
Long EC (1990) On demonstrating DNA intercalation. Acc Chem Res 9(23):271–273
Terenzi A, Barone G, Silvestri A et al (2009) The interaction of native calf thymus DNA with FeIII-dipyrido [3,2-a:2′,3′-c] phenazine[J]. J Inorg Biochem 103(1):1–9
Hanifa B, Sirajuddin M, Kubicki M et al (2022) Three isomeric 4-[(n-bromophenyl) carbamoyl] butanoic acids (n=2, 3 and 4) as DNA intercalator: synthesis, physicochemical characterization, antimicrobial activity, antioxidant potential and in silico study. J Mol Struct 1262:133033
Scott RL (1956) Some comments on the Benesi–Hildebrand equation. Recl Trav Chim Pays-Bas 75:787–789
Hammond PR (1964) 85. The effect of experimental errors on Benesi–Hildebrand plots and on the inherent accuracy of the equation. J Chem Soc 479–484
Hanifa B, Sirajuddin M, Tiekink ERT et al (2022) Designing, physiochemical confirmation, evaluation of biological and in-silico potential of Triorganotin(IV) complexes. J Mol Struct 1260:132814
Sirajuddin M, Ali S, Tahir MN (2021) Organotin (IV) derivatives based on 2-((2-methoxyphenyl) carbamoyl) benzoic acid: synthesis, spectroscopic characterization, assessment of antibacterial, DNA interaction, anticancer and antileishmanial potentials. J Mol Struct 1229:129600
Sirajuddin M, Ali S, Shah NA et al (2012) Synthesis, characterization, biological screenings and interaction with calf thymus DNA of a novel azomethine 3-((3,5-dimethylphenylimino) methyl) benzene-1,2-diol. Spectrochim Acta Part A Mol Biomol Spectrosc 94:134–142
Gayathri S, Viswanathamurthi P, Thuslim V et al (2022) Synthesis, structural, DNA/protein binding and cytotoxic studies of copper(I) ∝-diimine hydrazone complexes. Inorg Chim Acta 533:120780
Andrews SS, Tretton J (2020) Physical principles of circular dichroism. J Chem Educ 97:4370–4376
Norden B, Kurucsev T (1994) Analysing DNA complexes by circular and linear dichroism. J Mol Recognit 7:141–155
Meenongwa A, Brissos RF, Soikum C et al (2016) Effects of N, N-heterocyclic ligands on the in vitro cytotoxicity and DNA interactions of copper(II) chloride complexes from amidino-O-methylurea ligands. New J Chem 40:5861–5876
Ribeiro AG, Almeida SMVD, de Oliveira JF et al (2019) Novel 4-quinoline-thiosemicarbazone derivatives: synthesis, antiproliferative activity, in vitro and in silico biomacromolecule interaction studies and topoisomerase inhibition. Eur J Med Chem 182:111592
Kong D, Wang J, Zhu L et al (2008) Oxidative DNA cleavage by Schiff base tetraazamacrocyclic oxamido nickel(II) complexes. J Inorg Biochem 102:824–832
Garbett NC, Ragazzon PA, Chaires JB (2007) Circular dichroism to determine binding mode and affinity of ligand–DNA interactions. Nat Protoc 2:3166–3172
Baase WA, Johnson W Jr (1979) Grcular dichroism and DNA secondary structure. Nucleic Acids Res 6:797–814
Kashanian S, Askari S, Ahmadi F et al (2008) In vitro study of DNA interaction with clodinafop-propargyl herbicide. DNA Cell Biol 27:581–586
Zhang L, Liu Y, Hu X et al (2020) Studies on interactions of pentagalloyl glucose, ellagic acid and gallic acid with bovine serum albumin: a spectroscopic analysis. Food Chem 324:126872
Scognamiglio PL, Vicidomini C, Fontanella F et al (2022) Protein binding of benzofuran derivatives: a CD spectroscopic and in silico comparative study of the effects of 4-nitrophenyl functionalized benzofurans and benzodifurans on BSA protein structure. Biomolecules 12:262
Gospodarczyk W, Szutkowski K, Kozak M (2014) Interaction of bovine serum albumin (BSA) with novel Gemini surfactants studied by synchrotron radiation scattering (SR-SAXS), circular dichroism (CD), and nuclear magnetic resonance (NMR). J Phys Chem B 118:8652–8661
Zhu ZM, Zhang WJ (2021) Spectroscopic analysis of the interaction between the antiparasitic drug nitazoxanide and bovine serum albumin. Iran J Sci Technol Trans A Sci 45:867–873
Seng H, Von S, Tan K et al (2010) Crystal structure, DNA binding studies, nucleolytic property and topoisomerase I inhibition of zinc complex with 1,10-phenanthroline and 3-methyl-picolinic acid. Biometals 23:99–118
Seeliger D, de Groot BL (2010) Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des 24:417–422
Bheemarasetti M, Palakuri K, Raj S et al (2018) Novel Schiff base metal complexes: synthesis, characterization, DNA binding, DNA cleavage and molecular docking studies. J Iran Chem Soc 15:1377–1389
Chaurasia M, Tomar D, Chandra S (2019) Synthesis, spectral characterization, and DNA binding studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes of Schiff base 2-((1H–1,2,4-triazol-3-ylimino)methyl)-5-methoxyphenol. J Mol Struct 1179:431–442
Haribabu J, Alajrawy OI, Jeyalakshmi K et al (2021) N-substitution in isatin thiosemicarbazones decides nuclearity of Cu(II) complexes—spectroscopic, molecular docking and cytotoxic studies. Spectrochim Acta Part A Mol Biomol Spectrosc 246:118963
Haribabu J, Jeyalakshmi K, Arun Y et al (2015) Synthesis, DNA/protein binding, molecular docking, DNA cleavage and in vitro anticancer activity of nickel(II) bis(thiosemicarbazone) complexes. RSC Adv 5:46031–46049
Haribabu J, Jeyalakshmi K, Arun Y et al (2017) Synthesis of Ni(II) complexes bearing indole-based thiosemicarbazone ligands for interaction with biomolecules and some biological applications. J Biol Inorg Chem 22:461–480
GomathiSankareswari V, Vinod D, Mahalakshmi A et al (2014) Interaction of oxovanadium(IV)–salphen complexes with bovine serum albumin and their cytotoxicity against cancer. Dalton Trans 43:3260–3272
Funding
This study was funded by National Natural Science Foundation of China (Nos. 21261002, 31660251, 31860245, 31960203).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, B., Sun, D., Wang, S. et al. Nickel chloride complexes with substituted 4′-phenyl-2′,2′:6′,2″-terpyridine ligands: synthesis, characterization, anti-proliferation activity and biomolecule interactions. J Biol Inorg Chem 28, 627–641 (2023). https://doi.org/10.1007/s00775-023-02011-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-023-02011-3