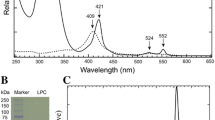
Abstract
In this study, we investigate the thermodynamic mechanisms by which electron transfer proteins adapt to environmental temperature by directly comparing the redox properties and folding stability of a psychrophilic cytochrome c and a mesophilic homolog. Our model system consists of two cytochrome c6 proteins from diatoms: one adapted specifically to polar environments, the other adapted generally to surface ocean environments. Direct electrochemistry shows that the midpoint potential for the mesophilic homolog is slightly higher at all temperatures measured. Cytochrome c6 from the psychrophilic diatom unfolds with a melting temperature 10.4 °C lower than the homologous mesophilic cytochrome c6. Changes in free energy upon unfolding are identical, within error, for the psychrophilic and mesophilic protein; however, the chemical unfolding transition of the psychrophilic cytochrome c6 is more cooperative than for the mesophilic cytochrome c6. Substituting alanine residues found in the mesophile with serine found in corresponding positions of the psychrophile demonstrates that burial of the polar serine both decreases the thermal stability and decreases the midpoint potential. The mutagenesis data, combined with differences in the m-value of chemical denaturation, suggest that differences in solvent accessibility of the hydrophobic core underlie the adaptation of cytochrome c6 to differing environmental temperature.
Graphic abstract






Similar content being viewed by others
Abbreviations
- cyt c :
-
Cytochrome c
- Fc:
-
Fragilariopsis cylindrus
- Tp:
-
Thalassiosira pseudonana
- PGE:
-
Pyrolytic graphite edge
- Gdn:
-
Guanidine HCl
- E mid :
-
Midpoint potential
References
Struvay C, Feller G (2012) Optimization to low temperature activity in psychrophilic enzymes. Int J Mol Sci 13:11643–11665. https://doi.org/10.3390/ijms130911643
Casanueva A, Tuffin M, Cary C, Cowan DA (2010) Molecular adaptations to psychrophily: the impact of “omic” technologies. Trends Microbiol 18:374–381. https://doi.org/10.1016/j.tim.2010.05.002
Gianese G, Bossa F, Pascarella S (2002) Comparative structural analysis of psychrophilic and meso- and thermophilic enzymes. Proteins Struct Funct Genet 47:236–249. https://doi.org/10.1002/prot.10084
Marcus RA, Sutin N (1985) Electron transfers in chemistry and biology. BBA Rev Bioenerg 811:265–322. https://doi.org/10.1016/0304-4173(85)90014-X
Worrall JAR, Schlarb-Ridley BG, Reda T et al (2007) Modulation of heme redox potential in the cytochrome c6 family. J Am Chem Soc 129:9468–9475. https://doi.org/10.1021/ja072346g
Schnackenberg J, Than M, Mann K et al (1999) Amino acid sequence, crystallization and structure determination of reduced and oxidized cytochrome c6 from the green alga Scenedesmus obliquus. J Mol Biol 290:1019–1030
Battistuzzi G, Borsari M, Sola M, Francia F (1997) Redox thermodynamics of the native and alkaline forms of eukaryortic and bacterial class I cytochromes c. Biochemistry 36:16247–16258. https://doi.org/10.1021/bi971535g
Terui N, Tachiiri N, Matsuo H et al (2003) Relationship between redox function and protein stability of cytochromes c. J Am Chem Soc 125:13650–13651. https://doi.org/10.1021/ja035682f
Armbrust EV, Berges JA, Bowler C et al (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306:79–86. https://doi.org/10.1126/science.1101156
Mock T, Otillar RP, Strauss J et al (2017) Evolutionary genomics of the cold-adapted diatom Fragilariopsis cylindrus. Nature 541:536–540. https://doi.org/10.1038/nature20803
Berges JA, Varela DE, Harrison PJ (2002) Effects of temperature on growth rate, cell composition and nitrogen metabolism in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). Mar Ecol Prog Ser 225:139–146. https://doi.org/10.3354/meps225139
Baek SH, Jung SW, Shin K (2011) Effects of temperature and salinity on growth of Thalassiosira pseudonana (Bacillariophyceae) isolated from ballast water. J Freshw Ecol 26:547–552. https://doi.org/10.1080/02705060.2011.582696
Ferguson RL, Collier A, Meete DA (1976) Growth response of Thalassiosira pseudonana Hasle and Heimdal Clone 3H to illumination temperature and nitrogen source. Chesap Sci 17:148–158
Lizotte MP (2001) The contributions of sea ice algae to antarctic marine primary production. Am Zool 41:57–73. https://doi.org/10.1668/0003-1569(2001)041[0057:TCOSIA]2.0.CO;2
Lyon BR, Mock T (2014) Polar microalgae: new approaches towards understanding adaptations to an extreme and changing environment. Biology (Basel) 3:56–80. https://doi.org/10.3390/biology3010056
Mock T, Valentin K (2004) Photosynthesis and cold acclimation: Molecular evidence from a polar diatom. J Phycol 40:732–741. https://doi.org/10.1111/j.1529-8817.2004.03224.x
Kilian O, Kroth PG (2005) Identification and characterization of a new conserved motif within the presequence of proteins targeted into complex diatom plastids. Plant J 41:175–183. https://doi.org/10.1111/j.1365-313X.2004.02294.x
Gruber A, Rocap G, Kroth PG et al (2015) Plastid proteome prediction for diatoms and other algae with secondary plastids of the red lineage. Plant J 81:519–528. https://doi.org/10.1111/tpj.12734
Nordberg H, Cantor M, Dusheyko S et al (2014) The genome portal of the Department of Energy Joint Genome Institute: 2014 updates. Nucleic Acids Res 42:D26–31. https://doi.org/10.1093/nar/gkt1069
Dyrløv Bendtsen J, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–795. https://doi.org/10.1016/J.JMB.2004.05.028
Arslan E, Schulz H, Zufferey R et al (1998) Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem Biophys Res Commun 747:744–747
Berry EA, Trumpower BL (1987) Simultaneous determination of hemes a, b and c from pyridine hemochrome spectra. Anal Biochem 161:1–15
Fourmond V (2016) QSoas: a versatile software for data analysis. Anal Chem 88:5050–5052. https://doi.org/10.1021/acs.analchem.6b00224
John DM, Weeks KM (2000) van’t Hoff enthalpies without baselines. Protein Sci 9:1416–1419. https://doi.org/10.1110/ps.9.7.1416
Santoro MM, Bolen DW (1988) Unfolding free energy changes determined by the linear extrapolation method. 1. Unfolding of phenylmethanesulfonyl alpha-chymotrypsin using different denaturants. Biochemistry 27:8063–8068. https://doi.org/10.1021/bi00421a014
Knapp JA, Pace CN (1974) Guanidine hydrochloride and acid denaturation of horse, cow, and Candida krusei cytochromes c. Biochemistry 13:1289–1294
Lojou É, Bianco P (2004) Membrane electrodes for protein and enzyme electrochemistry. Electroanalysis 16:1113–1121. https://doi.org/10.1002/elan.200403001
Correia dos Santos MM, Paes de Sousa PM, Simões Gonçalves ML et al (2003) Electrochemical studies on small electron transfer proteins using membrane electrodes. J Electroanal Chem 541:153–162. https://doi.org/10.1016/S0022-0728(02)01427-4
Ye T, Kaur R, Senguen FT et al (2008) Methionine ligand lability of type I cytochromes c: detection of ligand loss using protein film voltammetry. J Am Chem Soc 130:6682–6683. https://doi.org/10.1021/ja801071n
Levin BD, Can M, Bowman SEJ et al (2011) Methionine ligand lability in monohome cytochromes c: an electrochemical study. J Phys Chem B 115:11718–11726
Pettigrew GW, Moore GR (1987) Cytochromes c: biological aspects. Springer, Berlin
Cho YS, Wang QJ, Krogmann D, Whitmarsh J (1999) Extinction coefficients and midpoint potentials of cytochrome c6 from the cyanobacteria Arthrospira maxima, Microcystis aeruginosa, and Synechocystis 6803. Biochim Biophys Acta Bioenerg 1413:92–97. https://doi.org/10.1016/S0005-2736(99)00124-8
Bialek W, Nelson M, Tamiola K et al (2008) Deeply branching c6-like cytochromes of cyanobacteria. Biochemistry 47:5515–5522. https://doi.org/10.1021/bi701973g
Campos AP, Aguiar AP, Hervas M et al (1993) Cytochrome c6 from Monoraphidium braunii: a cytochrome with an unusual heme axial coordination. Eur J Biochem 216:329–341. https://doi.org/10.1111/j.1432-1033.1993.tb18150.x
Dikiy A, Carpentier W, Vandenberghe I et al (2002) Structural basis for the molecular properties of cytochrome c6. Biochemistry 41:14689–14699
Sokolovskaya OM, Magyar JS, Buzzeo MC (2014) Electrochemical behavior of cytochrome c552 from a psychrophilic microorganism. J Phys Chem C 118:18829–18835. https://doi.org/10.1021/jp501146e
D’Amico S, Marx JC, Gerday C, Feller G (2003) Activity-stability relationships in extremophilic enzymes. J Biol Chem 278:7891–7896. https://doi.org/10.1074/jbc.M212508200
Satoh T, Itoga A, Isogai Y et al (2002) Increasing the conformational stability by replacement of heme axial ligand in c-type cytochrome. FEBS Lett 531:543–547. https://doi.org/10.1016/S0014-5793(02)03615-3
Akazaki H, Kawai F, Chida H et al (2008) Cloning, expression and purification of cytochrome c6 from the brown alga Hizikia fusiformis and complete X-ray diffraction analysis of the structure. Acta Crystallogr Sect F Struct Biol Cryst Commun 64:674–680. https://doi.org/10.1107/S1744309108017752
Lange C, Luque I, Hervás M et al (2005) Role of the surface charges D72 and K8 in the function and structural stability of the cytochrome c6 from Nostoc sp. PCC 7119. FEBS J 272:3317–3327. https://doi.org/10.1111/j.1742-4658.2005.04747.x
Mason JM, Bendall DS, Howe CJ, Worrall JAR (2012) The role of a disulfide bridge in the stability and folding kinetics of Arabidopsis thaliana cytochrome c6A. Biochim Biophys Acta Proteins Proteom 1824:311–318. https://doi.org/10.1016/j.bbapap.2011.10.015
Kim DE, Chivian D, Baker D (2004) Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. https://doi.org/10.1093/nar/gkh468
Fraczkiewicz R, Braun W (1998) Exact and efficient analytical calculation of the accessible surface areas and their gradients for macromolecules. J Comput Chem 19:319–333
Hasegawa J, Uchiyama S, Tanimoto Y et al (2000) Selected mutations in a mesophilic cytochrome c confer the stability of a thermophilic counterpart. J Biol Chem 275:37824–37828. https://doi.org/10.1074/jbc.M005861200
Wen X, Patel KM, Russell BS, Bren KL (2007) Effects of heme pocket structure and mobility on cytochrome c stability. Biochemistry 46:2537–2544. https://doi.org/10.1021/bi602380v
Ratcliff K, Corn J, Marqusee S (2009) Structure, stability, and folding of ribonuclease H1 from the moderately thermophilic Chlorobium tepidum: comparison with thermophilic and mesophilic homologues. Biochemistry 48:5890–5898. https://doi.org/10.1021/bi900305p
Myers JK, Nick Pace C, Martin Scholtz J (1995) Denaturant m values and heat capacity changes: relation to changes in accessible surface areas of protein unfolding. Protein Sci 4:2138–2148. https://doi.org/10.1002/pro.5560041020
Pickett SD, Sternberg MJE (1993) Empirical scale of side-chain conformational entropy in protein folding. J Mol Biol 231:825–839. https://doi.org/10.1006/jmbi.1993.1329
Hosseinzadeh P, Lu Y (2016) Design and fine-tuning redox potentials of metalloproteins involved in electron transfer in bioenergetics. Biochim Biophys Acta Bioenerg 1857:557–581. https://doi.org/10.1016/j.bbabio.2015.08.006
Fantuzzi A, Sadeghi S, Valetti F et al (2002) Tuning the reduction potential of engineered cytochrome c-553. Biochemistry 41:8718–8724. https://doi.org/10.1021/bi025759x
Tezcan FA, Winkler JR, Gray HB (1998) Effects of ligation and folding on reduction potentials of heme proteins. J Am Chem Soc 120:13383–13388. https://doi.org/10.1021/ja982536e
Bortolotti CA, Amadei A, Aschi M et al (2012) The reversible opening of water channels in cytochrome c modulates the heme iron reduction potential. J Am Chem Soc 134:13670–13678. https://doi.org/10.1021/ja3030356
Acknowledgements
The authors thank the M. J. Murdock Charitable Trust for funding through the Murdock College Research Program for Natural Sciences. We thank the Seattle University College of Science and Engineering for summer support. Seattle University students enrolled in CHEM 1910 collected key preliminary cyclic voltammetry data for this study.
Funding
This study was funded by the M. J. Murdock Charitable Trust Murdock College Research Program for Natural Sciences (2015302:MNL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wilson, M., Tillery, L., Tabaie, E. et al. Alanine to serine substitutions drive thermal adaptation in a psychrophilic diatom cytochrome c6. J Biol Inorg Chem 25, 489–500 (2020). https://doi.org/10.1007/s00775-020-01777-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-020-01777-0