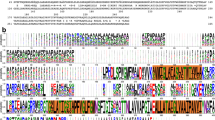
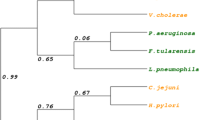
Abstract
DNA-binding protein from starved cells (Dps)-like proteins are key factors involved in oxidative stress protection in bacteria. They bind and oxidize iron, thus preventing the formation of harmful reactive oxygen species that can damage biomolecules, particularly DNA. Dps-like proteins are composed of 12 identical subunits assembled in a spherical structure with a hollow central cavity. The iron oxidation occurs at 12 intersubunit sites located at dimer interfaces. Streptococcus pyogenes Dps-like peroxide resistance protein (Dpr) has been previously found to protect the catalase-lacking S. pyogenes bacterium from oxidative stress. We have determined the crystal structure of S. pyogenes Dpr, the second Dpr structure from a streptococcal bacterium, in iron-free and iron-bound forms at 2.0- and 1.93-Å resolution, respectively. The iron binds to well-conserved sites at dimer interfaces and is coordinated directly to Asp77 and Glu81 from one monomer, His50 from a twofold symmetry-related monomer, a glycerol molecule, and a water molecule. Upon iron binding, Asp77 and Glu81 change conformation. Site-directed mutagenesis of active-site residues His50, His62, Asp66, Asp77, and Glu81 to Ala revealed a dramatic decrease in iron incorporation. A short helix at the N-terminal was found in a different position compared with other Dps-like proteins. Two types of pores were identified in the dodecamer. Although the N-terminal pore was found to be similar to that of other Dps-like proteins, the C-terminal pore was found to be blocked by bulky Tyr residues instead of small residues present in other Dps-like proteins.






Similar content being viewed by others
Abbreviations
- Dps:
-
DNA-binding protein from starved cells
- FOC:
-
Ferroxidase center
- PDB:
-
Protein Data Bank
- RMSD:
-
Root-mean-square deviation
- SpDpr:
-
Streptococcus pyogenes DNA-binding protein from starved cells like peroxide resistance protein
- SsDpr:
-
Streptococcus suis DNA-binding protein from starved cells like peroxide resistance protein
References
Storz G, Imlay JA (1999) Curr Opin Microbiol 2:188–194
Imlay JA (2008) Annu Rev Biochem 77:755–776
Pulliainen AT, Hytonen J, Haataja S, Finne J (2008) J Bacteriol 190:3225–3235
Bozzi M, Mignogna G, Stefanini S, Barra D, Longhi C, Valenti P, Chiancone E (1997) J Biol Chem 272:3259–3265
Roy S, Gupta S, Das S, Sekar K, Chatterji D, Vijayan M (2003) Acta Crystallogr D Biol Crystallogr 59:2254–2256
Almirón M, Link AJ, Furlong D, Kolter R (1992) Genes Dev 6:2646–2654
Chen L, Helmann JD (1995) Mol Microbiol 18:295–300
Halsey TA, Vazquez-Torres A, Gravdahl DJ, Fang FC, Libby SJ (2004) Infect Immun 72:1155–1158
Nicodeme M, Perrin C, Hols P, Bracquart P, Gaillard JL (2004) Curr Microbiol 48:51–56
Ramsay B, Wiedenheft B, Allen M, Gauss GH, Lawrence CM, Young M, Douglas T (2006) J Inorg Biochem 100:1061–1068
Stillman TJ, Upadhyay M, Norte VA, Sedelnikova SE, Carradus M, Tzokov S, Bullough PA, Shearman CA, Gasson MJ, Williams CH, Artymiuk PJ, Green J (2005) Mol Microbiol 57:1101–1112
Roy S, Gupta S, Das S, Sekar K, Chatterji D, Vijayan M (2004) J Mol Biol 339:1103–1113
Ceci P, Cellai S, Falvo E, Rivetti C, Rossi G, Chiancone E (2004) Nucleic Acids Res 32:5935–5944
Bhattacharyya G, Grove A (2007) J Biol Chem 282:11921–11930
Evans DJ Jr, Evans DG, Takemura T, Nakano H, Lampert HC, Graham DY, Granger DN, Kvietys PR (1995) Infect Immun 63:2213–2220
Li X, Pal U, Ramamoorthi N, Liu X, Desrosiers DC, Eggers CH, Anderson JF, Radolf JD, Fikrig E (2007) Mol Microbiol 63:694–710
Harrison PM, Arosio P (1996) Biochim Biophys Acta 1275:161–203
Carrondo MA (2003) EMBO J 22:1959–1968
Tsou CC, Chiang-Ni C, Lin YS, Chuang WJ, Lin MT, Liu CC, Wu JJ (2008) Infect Immun 76:4038–4045
Leslie AG (2006) Acta Crystallogr D Biol Crystallogr 62:48–57
Collaborative Computational Project Number 4 (1994) Acta Crystallogr D Biol Crystallogr 50:760–763
Stein N (2008) J Appl Crystallogr 41:641–643
Chen YW, Dodson EJ, Kleywegt GJ (2000) Structure 8:R213–R220
Kauko A, Haataja S, Pulliainen AT, Finne J, Papageorgiou AC (2004) J Mol Biol 338:547–558
McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ (2007) J Appl Crystallogr 40:658–674
Morris RJ, Perrakis A, Lamzin VS (2003) Methods Enzymol 374:229–244
Murshudov GN, Vagin AA, Dodson EJ (1997) Acta Crystallogr D Biol Crystallogr 53:240–255
Emsley P, Cowtan K (2004) Acta Crystallogr D Biol Crystallogr 60:2126–2132
Krissinel EB, Winn MD, Ballard CC, Ashton AW, Patel P, Potterton EA, McNicholas SJ, Cowtan KD, Emsley P (2004) Acta Crystallogr D Biol Crystallogr 60:2250–2255
Romao CV, Mitchell EP, McSweeney S (2006) J Biol Inorg Chem 11:891–902
Grant RA, Filman DJ, Finkel SE, Kolter R, Hogle JM (1998) Nat Struct Biol 5:294–303
Ilari A, Stefanini S, Chiancone E, Tsernoglou D (2000) Nat Struct Biol 7:38–43
Ren B, Tibbelin G, Kajino T, Asami O, Ladenstein R (2003) J Mol Biol 329:467–477
Roy S, Saraswathi R, Gupta S, Sekar K, Chatterji D, Vijayan M (2007) J Mol Biol 370:752–767
Gauss GH, Benas P, Wiedenheft B, Young M, Douglas T, Lawrence CM (2006) Biochemistry 45:10815–10827
Zanotti G, Papinutto E, Dundon W, Battistutta R, Seveso M, Giudice G, Rappuoli R, Montecucco C (2002) J Mol Biol 323:125–130
Papinutto E, Dundon WG, Pitulis N, Battistutta R, Montecucco C, Zanotti G (2002) J Biol Chem 277:15093–15098
Thumiger A, Polenghi A, Papinutto E, Battistutta R, Montecucco C, Zanotti G (2006) Proteins 62:827–830
Zeth K, Offermann S, Essen LO, Oesterhelt D (2004) Proc Natl Acad Sci USA 101:13780–13785
Kim SG, Bhattacharyya G, Grove A, Lee YH (2006) J Mol Biol 361:105–114
Cuypers MG, Mitchell EP, Romao CV, McSweeney SM (2007) J Mol Biol 371:787–799
Kauko A, Pulliainen AT, Haataja S, Meyer-Klaucke W, Finne J, Papageorgiou AC (2006) J Mol Biol 364:97–109
Ceci P, Ilari A, Falvo E, Chiancone E (2003) J Biol Chem 278:20319–20326
Franceschini S, Ceci P, Alaleona F, Chiancone E, Ilari A (2006) FEBS J 273:4913–4928
Pulliainen AT, Kauko A, Haataja S, Papageorgiou AC, Finne J (2005) Mol Microbiol 57:1086–1100
Ilari A, Ceci P, Ferrari D, Rossi GL, Chiancone E (2002) J Biol Chem 277:37619–37623
Yamamoto Y, Poole LB, Hantgan RR, Kamio Y (2002) J Bacteriol 184:2931–2939
Acknowledgments
We thank the Academy of Finland for financial support (grant no. 121278 to A.C.P.), the National Science Council, Taiwan, for grants NSC96-2320-B-006-008 and NSC97-2311-B-006-004-MY3 (to J.J.W.), and the European Molecular Biology Laboratory, Hamburg outstation, for access to synchrotron radiation facilities. Support from the European Community—Research Infrastructure Action Under the FP6 (structuring the European Research Area Programme contract number RII3/CT/2004/5060008) is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haikarainen, T., Tsou, CC., Wu, JJ. et al. Crystal structures of Streptococcus pyogenes Dpr reveal a dodecameric iron-binding protein with a ferroxidase site. J Biol Inorg Chem 15, 183–194 (2010). https://doi.org/10.1007/s00775-009-0582-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-009-0582-9