Abstract
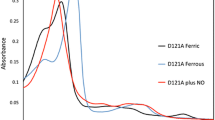
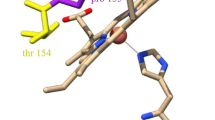
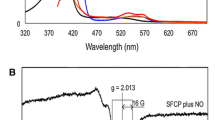
We have cloned and expressed the cycP gene encoding cytochrome c′ from Alcaligenes xylosoxidans and generated mutations in Arg-124 and Phe-59, residues close to the haem, to probe their involvement in modulating the unusual spin-state equilibrium of the haem Fe and the unique proximal mode of binding of NO to form a stable five-coordinate adduct. Arg-124 is located in the proximal pocket of the haem and forms a hydrogen bond to the stable five-coordinated bound NO. Phe-59 provides steric hindrance at the distal face where NO binds initially to form a six-coordinate adduct. Optical spectroscopy showed altered electronic properties of the oxidised haem centre resulting from the mutations of both residues. The high affinity of the ferrous proteins for NO remained unchanged and all of the mutational variants formed a stable five-coordinate NO species (λ Soret 395 nm) in the presence of stoichiometric concentrations of NO. However, the kinetics of the reactivity towards NO were altered, with mutation of the distal Phe-59 residue resulting in the transient six-coordinate distally bound NO adduct (λ Soret 415 nm) not being detected. Surprisingly, substitution of the proximal residue Arg-124 with Phe, Ala, Gln or Glu also resulted in the six-coordinate adduct not being detected, showing that this proximal residue also modulates reactivity towards NO on the opposite haem face. In contrast, the R124L substitution retained the property of the native protein in the initial formation of a six-coordinate NO adduct, a finding of functional importance since a Lys or an Arg residue is invariant in these proteins.





Similar content being viewed by others
References
Rogers KR (1999) Curr Opin Chem Biol 8:158–167
Gilles-Gonzalez M-A, Gonzalez G (2005) J Inorg Biochem 99:1–22
Karow DS, Pan D, Tran R, Pellicena P, Presley A, Mathies RA, Marletta MA (2004) Biochemistry 43:10203–10211
Meyer TE, Kamen MD (1982) Adv Protein Chem 35:105–212
Romão MJ, Archer M (2001) Handbook of metalloproteins. Wiley, Chichester, pp 44–54
Weiss R, Gold A, Terner J (2006) Chem Rev 106:2550–2579
Lawson DM, Stevenson CEM, Andrew CR, Eady RR (2000) EMBO J 19:5661–5671
Cross R, Aish J, Paston SJ, Poole RK, Moir JWB (2000) J Bacteriol 182:1442–1447
Choi PS, Grigoryants VM, Abruña HD, Scholes CP, Shapleigh JP (2005) J Bacteriol 187:4077–4085
Mayburd AL, Kassner RJ (2002) Biochemistry 41:11582–1159
Andrew CR, George SJ, Lawson DL, Eady RR (2002) Biochemistry 41:2353–2360
Makino R, Matsuda H, Obayashi E, Shiro Y, Izuka T, Hori H (1999) J Biol Chem 274:7714–7723
Zhao Y, Brandish PE, Ballou DP, Marletta MA (1999) Proc Natl Acad Sci USA 96:14753–14758
Maltempo MM (1974) J Chem Phys 61:2450–2457
Maltempo MM, Moss TH (1976) Q Rev Biophys 9:181–215
Weber PC (1982) Biochemistry 21:5116–5119
Kruglik SG, Lambry J-C, Cianetti S, Martin J-L, Eady RR, Andrew CR, Negrerie M (2006) J Biol Chem 282:5053–5062
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Arslan E, Schulz H, Zufferet R, Kunzler P, Thony-Meyer L (1998) Biomed Biophys Res Commun 251:744–747
Zacharia I, Deen W (2005) Ann Biomed Eng 33:214–222
Cusanovich MA, Tedro SM, Kamen MD (1970) Arch Biochem Biophys 141:557–570
Thony-Meyer L (1997) Microbiol Molec Biol Rev 337–376
Leon RG, Munier-Lehman H, Barzu O, Baudin-Creuza V, Pietri R, Lopez Garriga J, Cadilla CL (2004) Protein Expr Purif 38:184–195
Barbieri S (2007) PhD thesis, University of Wales, Bangor
Andrew CR, Kemper JL, Busche TL, Tiwari AM, Kecskes MC, Stafford JM, Croft LC, Lu S, Moenne-Loccoz P, Huston W, Moir JWB, Eady RR (2005) Biochemistry 44:8664–8672
Marti MA, Capece L, Crespo A, Doctotovich F, Estrin DA (2005) J Am Chem Soc 127:7721–7728
Yoshimura T (1985) Biochemistry 25:2436–2442
Strekas TC, Spiro TG (1974) Biochim Biophys Acta 351:237–245
George SJ, Andrew CR, Lawson DM, Thorneley RNF, Eady RR (2001) J Am Chem Soc 123:9683–9684
Yoshimura T, Suzuki S, Nakahara A, Iwasaki H, Masuko M, Matsubara T (1985) Biochim Biophys Acta 831:267–274
Yoshimura T (1985) Biochemistry 25:2436–2432
Dobbs JA, Anderson BF, Faber HR, Baker E (1996) Acta Crystallogr Sect D 52:356–361
Huston WH, Andrew CR, Servid AE, McKay AL, Leech AP, Butler CS, Moir JWB (2006) Biochemistry 45:4388–4395
Martin E, Berka V, Bogatenkova E, Murad F, Tsai A-L (2006) J Biol Chem 281:27836–27845
Acknowledgments
We would like to thank STFC Daresbury laboratory for provision of facilities and resources. S.B. received a studentship from the School of Chemistry, Bangor University. We thank members of the Molecular Biophysics Group for useful discussions and help throughout this work and Mark Ellis for help with the figures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbieri, S., Murphy, L.M., Sawers, R.G. et al. Modulation of NO binding to cytochrome c′ by distal and proximal haem pocket residues. J Biol Inorg Chem 13, 531–540 (2008). https://doi.org/10.1007/s00775-008-0341-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-008-0341-3