Abstract
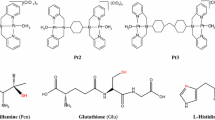
The substitution reactions of [PtCl(bpma)]+, [PtCl(gly-met-S,N,N)], [Pt(bpma)(H2O)]2+ and [Pt(gly-met-S,N,N)(H2O)]+ [where bpma is bis(2-pyridylmethyl)amine and gly-met-S,N,N is glycylmethionine] with l-methionine, glutathione and guanosine 5′-monophosphate (5′-GMP) were studied in aqueous solutions in 0.10 M NaClO4 under pseudo-first-order conditions as a function of concentration and temperature using UV–vis spectrophotometry. The reactions of the chloro complexes were followed in the presence of 10 mM NaCl and at pH ~ 5, whereas the reactions of the aqua complexes were studied at pH 2.5. The [PtCl(bpma)]+ complex is more reactive towards the chosen nucleophiles than [PtCl(gly-met-S,N,N)]. Also, the aqua complexes are more reactive than the corresponding chloro complexes. The activation parameters for all the reactions studied suggest an associative substitution mechanism. The reactions of [PtCl(bpma)]+ and [PtCl(gly-met-S,N,N)] with 5′-GMP were studied by using 1H NMR spectroscopy at 298 K. The pK a value of the [Pt(gly-met-S,N,N)(H2O)]+ complex is 5.95. Density functional theory calculations (B3LYP/LANL2DZp) show that in all cases guanine coordination to the L3Pt fragment (L3 is terpyridine, bpma, diethylenetriamine, gly-met-S,N,N) is much more favorable than the thioether-coordinated form. The calculations collectively support the experimentally observed substitution of thioethers from Pt(II) complexes by N7-GMP. This study throws more light on the mechanistic behavior of platinum antitumor complexes.










Similar content being viewed by others
References
Wang D, Lippard SJ (2005) Nat Rev Drug Discov 4:307–320
Lippert B (1999) Cisplatin: chemistry and biochemistry of a leading anticancer drug. Wiley, Zurich
Furtes MA, Alonso C, Pérez JM (2003) Chem Rev 103:645–662
Jakubec MA, Galanski M, Keppler BK (2003) Rev Physiol Biochem Pharmacol 146:1–53
Esposito BP, Najjar R (2002) Coord Chem Rev 232:137–149
Wong E, Giandomenico CM (1999) Chem Rev 99:2451–2466
Jamieson ER, Lippard SJ (1999) Chem Rev 99:2467–2498
van Zutphen S, Reedijk J (2005) Coord Chem Rev 249:2845– 2853
Reedijk J (1999) Chem Rev 99:2499–2510
Reedijk J (2003) Proc Natl Acad Sci USA 100:3611–3616
Zorbas H, Keppler BK (2005) Chembiochem 6:1157–1166
Dor RT (1996) Platinum and other metal coordination compounds in cancer chemotherapy. Plenum, New York, pp 131–154
Soldatović T, Bugarčić ŽD (2005) J Inorg Biochem 99:1472–1479
Bose RN, Moghaddas S, Weaver EL, Cox EH (1995) Inorg Chem 34:5878–5883
Barnham KJ, Djuran MI, Murdoch PDS, Sadler PJ (1994) J Chem Soc Chem Commun 6:721–722
Teuben JM, van Boom SSGE, Reedijk J (1977) J Chem Soc Dalton Trans 3979–3980
Barnham KJ, Guo Z, Sadler PJ (1996) J Chem Soc Dalton Trans 2867–2876
Barnham KJ, Djuran MI, Murdoch PdS, Ranford JD, Sadler PJ (1995) J Chem Soc Dalton Trans 3721–3276
Teuben JM, Zubiri MRI, Reedijk J (2000) Chem Soc Dalton Trans 369–372
Bugarčić ŽD, Soldatović T, Jelić R, Alguero B, Grandas A (2004) Dalton Trans 22:3869–3877
Bugarčić ŽD, Liehr G, van Eldik R (2002) J Chem Soc Dalton Trans 14:2825–2830
Bugarčić ŽD, Heinemann FW, van Eldik R (2004) Dalton Trans 2:279–286
Hofmann A, Jaganyi D, Munro QO, Liehr G, van Eldik R (2003) Inorg Chem 42:1688–1700
Guo X, Wang X, Ding J, Lin L, Li Y, Guo Z (2006) Inorg Chem Commun 9:722–726
Zhang J, Wang X, Tu C, Lin J, Ding J, Lin L, Wang Z, He C, Yan C, You X, Guo Z (2003) J Med Chem 46:3502–3507
Summa N, Schiessl W, Puchta P, van Eikema Hommes N, van Eldik R (2006) Inorg Chem 45:2948–2959
Summa N, Soldatović T, Dahlenburg L, Bugarčić ŽD, van Eldik R (2007) J Biol Inorg Chem 12:461–475
Jaganui D, Tiba F, Munro OQ, Petrović B, Bugarčić ŽD (2006) Dalton Trans 2943–2949
Bugarčić ŽD, Ilić D, Djuran M (2001) Aust J Chem 54:237–240
Appleton TG, Hall JR, Ralph SF, Thompson CSM (1984) Inorg Chem 23:3521–3525
Mikkelsen K, Nielsen SO (1960) J Phys Chem 64:632–637
Becke AD (1993) J Phys Chem 97:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) J Phys Chem 98:11623–11627
Dunning TH Jr, Hay PJ (1976) Mod Theor Chem 3:1–28
Hay PJ, Wadt WR (1985) J Chem Phys 82:270–283
Hay PJ, Wadt WR (1985) J Chem Phys 82:284–298
Hay PJ, Wadt WR (1985) J Chem Phys 82:299–310
Huzinaga S (ed) (1984) Gaussian basis sets for molecular calculations. Elsevier, Amsterdam
Schiessl W, Puchta R, Bugarčić ŽD, Heinemann FW, van Eldik R (2007) Eur J Inorg Chem:1390–1404
Galle M, Puchta R, van Eikema Hommes NJR, van Eldik R (2006) Z Phys Chem 220:511–523
Puchta R, Meier R, van Eikema Hommes NJR, van Eldik R (2006) Eur J Inorg Chem 4063–4067
Scheurer A, Maid H, Hampel F, Saalfrank RW, Toupet L, Mosset P, Puchta R, van Eikema Hommes NJR (2005) Eur J Org Chem 2566–2574
Illner P, Zahl A, Puchta R, van Eikema Hommes N, Wasserscheid P, van Eldik R (2005) J Organomet Chem 690:3567–3576
Weber ChF, Puchta R, van Eikema Hommes N, Wasserscheid P, van Eldik R (2005) Angew Chem 117:6187–6192
Weber ChF, Puchta R, van Eikema Hommes N, Wasserscheid P, van Eldik R (2005) Angew Chem Int Ed Engl 44:6033–6038
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision C.02. Gaussian, Wallingford
Barone V, Cossi M (1998) J Phys Chem A 102:1995–2001
Cossi M, Rega N, Scalmani G, Barone V (2003) J Comput Chem 24:669–681
Tobe ML, Burgess J (1999) Inorganic reaction mechanisms, Addison Wesley Longman, Harlow, chap 3
Frey U, Ranford JD, Sadler PJ (1993) Inorg Chem 32:1333–1340
Lempers ELM, Reedijk J (1991) Adv Inorg Chem 37:175–217
van Boom SSGE, Chen BW, Tauben JM, Reedijk J (1999) Inorg Chem 38:1450–1455
Jaganyi D, Hofmann A, van Eldik R (2001) Angew Chem Int Ed Engl 40:1680–1683
Jaganyi D, Tiba F (2003) Trans Met Chem 28:803–807
Hubbard CD, van Eldik R (2007) J Coord Chem 60:1–51
Lippert B (1999) Cisplatin: chemistry and biochemistry of a leading anticancer drug. Wiley, Zurich, pp 183–221
Arpalahti J, Lippert B (1990) Inorg Chem 29:104–110
Caradonna JP, Lippard SJ (1988) Inorg Chem 27:1454–1466
Mikola M, Kilika KD, Arpalahti J (2000) Chem Eur J 3404–3413
Laidler KJ (1987) Chemical kinetics, 3rd edn. Harper and Row, New York, p 22
Volckova E, Dudones LP, Bose RN (2002) Pharm Res 19:124–131
Acknowledgements
The authors gratefully acknowledge financial support from the Ministry of Science and Technology, Republic of Serbia (project no. 142008) and the Deutsche Forschungsgemeinschaft (SFB 583 “Redox-active metal complexes”). We thank Tim Clark for hosting this work at the CCC and the Regionales Rechenzentrum Erlangen (RRZE) for a generous allotment of computer time.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bugarčić, Ž.D., Rosić, J., Petrović, B. et al. Kinetics and mechanism of the substitution reactions of [PtCl(bpma)]+, [PtCl(gly-met-S,N,N)] and their aqua analogues with l-methionine, glutathione and 5′-GMP. J Biol Inorg Chem 12, 1141–1150 (2007). https://doi.org/10.1007/s00775-007-0283-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-007-0283-1