Abstract
Introduction
Osteoarthritis (OA) compromises patients’ quality of life and requires further study. Although miR-92a-3p was reported to possess chondroprotective effects, the underlying mechanism requires further clarification. The objectives of this study were to elucidate the mechanism by which miR-92a-3p alleviates OA and to examine the efficacy of shRNA-92a-3p, which was designed based on mature miR-92a-3p.
Materials and methods
TargetScan and luciferase reporter assay were used to predict the target of miR-92a-3p. Adipose-derived stem cells (ADSCs) were transfected with miR-92a-3p/miR-NC mimic for the analysis of chondrogenic biomarkers and SMAD proteins. ADSCs and osteoarthritic chondrocytes were transduced with shRNA-92a-3p for the analysis of chondrogenic biomarkers and SMAD proteins. OA was surgically induced in C57BL/6JJcl mice, and ADSCs with/without shRNA-92a-3p transduction were intra-articularly injected for the assessment of cartilage damage.
Results
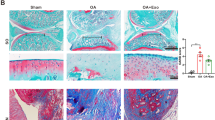
SMAD6 and SMAD7 were predicted as direct targets of miR-92a-3p by TargetScan and luciferase reporter assay. Transfection of the miR-92a-3p mimic resulted in a decrease in SMAD6 and SMAD7 levels and an increase in phospho-SMAD2/3, phospho-SMAD1/5/9, SOX9, collagen type II, and aggrecan levels in ADSCs. Furthermore, shRNA-92a-3p decreased SMAD6 and SMAD7 levels, and increased phospho-SMAD2/3, phospho-SMAD1/5/9, SOX9, collagen type II, and aggrecan levels in ADSCs and osteoarthritic chondrocytes. Additionally, ADSC-shRNA-92a-3p-EVs reduced the rate of decrease of SOX9, collagen type II, and aggrecan in osteoarthritic chondrocytes. In mice with surgically induced OA, shRNA-92a-3p-treated ADSCs alleviated cartilage damage more effectively than nontreated ADSCs.
Conclusions
miR-92a-3p and shRNA-92a-3p exhibit therapeutic effects in treating OA by targeting SMAD6 and SMAD7, thereby enhancing TGF-β signaling.






Similar content being viewed by others
References
Richard FL, Steven RG, Carla RS, Mary BG (2012) Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 64:1697–1707
Mary BG, Steven RG (2007) Osteoarthritis. J Cell Physiol 213:626–634
Maroudas AI (1976) Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature 260:808–809
Bollet AJ, Nance JL (1966) Biochemical findings in normal and osteoarthritic articular cartilage. II. Chondroitin sulfate concentration and chain length, water, and ash content. J Clin Invest 45:1170–1177
Kyung-Su P, Min-Jung P, Mi-La C, Seung-Ki K, Ji HJ, Hyeok-Jae K, Sung-Hwan P, Ho-Youn K (2009) Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis. Mod Rheumatol 19:581–589
Fulya B, Onur A, Merih O, Funda T, Ozge B, Ozkan A (2016) Effects of native type ii collagen treatment on knee osteoarthritis: a randomized controlled trial. Eurasian J Med 48:95–101
Chris K, Liwen C, Yao Jiong W, Albert JY, Burton BY (2002) Structure and function of aggrecan. Cell Res 12:19–32
Doege KJ, Sasaki M, Kimura T, Yamada Y (1991) Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem 266:894–902
Roughley P, Martens D, Rantakokko J, Alini M, Mwale F, Antoniou J (2006) The involvement of aggrecan polymorphism in degeneration of human intervertebral disc and articular cartilage. Eur Cell Mater 11:1–7
Berenbaum F (2013) Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 21:16–21
Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ (2015) Osteoarthritis. The Lancet 386:376–387
Yves-Marie P, Lars R, Rosanna F, Oliver P, Christophe D et al (2016) Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med 5:847–856
Chris HJ, Young GL, Won HS, Hyang K, Jee WC, Eui CJ, Ji EK, Hackjoon S, Ji SS, Il SS, Jeong CR, Sohee O, Kang SY (2014) Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells 32:1254–1266
Sun Q, Zhang L, Xu T, Ying J, Xia B, Jing H, Tong P (2018) Combined use of adipose derived stem cells and TGF-β3 microspheres promotes articular cartilage regeneration in vivo. Biotech Histochem 93:168–176
Li-Bo J, Soomin L, Yang W, Qin-Tong X, De-Hua M, Jian Z (2016) Adipose-derived stem cells induce autophagic activation and inhibit catabolic response to pro-inflammatory cytokines in rat chondrocytes. Osteoarthritis Cartilage 24:1071–1081
Victoria LJ, David JH (2014) The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol 28:5–15
Jai-Hong C, Chieh-Cheng H, Shan-Ling H, Wen-Yi C, Yi-No W, Chun-En AK, Tsai-Chin H, Li-Yen S, Shun-Wun J (2021) Adipose-derived mesenchymal stem cells-conditioned medium modulates the expression of inflammation induced bone morphogenetic protein-2, -5 and -6 as well as compared with shockwave therapy on rat knee osteoarthritis. Biomedicines 9:1399
Shuangpeng J, Guangzhao T, Xu L, Zhen Y, Fuxin W, Zhuang T, Bo H, Fu W, Kangkang Z, Zhiqiang S, Xiang S, Shuyun L, Weimin G, Quanyi G (2021) Research progress on stem cell therapies for articular cartilage regeneration. Stem Cells Int 2021:8882505
Xiaoting L, Yue D, Yuelin Z, Hung-Fat T, Qizhou L (2014) Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant 23:1045–1059
Liwen C, Edward ET, Philip YGW, Yaojiong W (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 3:e1886
Abir NM, Omar SET, Ashraf AS, Laila AR, Dina S, Abeer MES (2011) Homing and reparative effect of intra-articular injection of autologus mesenchymal stem cells in osteoarthritic animal model. BMC Musculoskelet Disord 12:259
Mokbel A, El-Tookhy O, Shamaa AA, Sabry D, Rashed L, Mostafa A (2011) Homing and efficacy of intra-articular injection of autologous mesenchymal stem cells in experimental chondral defects in dogs. Clin Exp Rheumatol 29:275–284
Jonathan CB, Gordana VN (2016) Should we use cells, biomaterials, or tissue engineering for cartilage regeneration? Stem Cell Res Ther 7:56
Sheng Z, Song C, Qing J, Ming P (2019) Determinants of stem cell lineage differentiation toward chondrogenesis versus adipogenesis. Cell Mol Life Sci 76:1653–1680
Tao W, Puwapong N, Christopher AS, Aixin C, Timothy EH, Susan JK (2019) Enhanced chondrogenesis from human embryonic stem cells. Stem cell research 39:101497
Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B (1998) The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am 80:1745–1757
Hongjun K, Shibi L, Jiang P, Qiang Y, Shuyun L, Li Z, Jingxiang H, Xiang S, Bin Z, Aiyuan W, Wenjing X, Quanyi G, Qing S (2015) In vivo construction of tissue-engineered cartilage using adipose-derived stem cells and bioreactor technology. Cell Tissue Bank 16:123–133
Caldwell KL, Wang J (2015) Cell-based articular cartilage repair: the link between development and regeneration. Osteoarthritis Cartilage 23:351–362
Richard WC, Erik JS (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136:642–655
Jun L, Ming-Liang J, Xue-Jun Z, Pei-Liang S, Hao W, Chen W, Hee-Jeong I (2017) MicroRNA-218-5p as a potential target for the treatment of human osteoarthritis. Mol Ther 25:2676–2688
Wei Z, Biao Z, Chi Z, Congfeng L, Yulin Z (2018) miR-373 regulates inflammatory cytokine-mediated chondrocyte proliferation in osteoarthritis by targeting the P2X7 receptor. FEBS Open Bio 8:325–331
Changhe H, Zibo Y, Yan K, Ziji Z, Ming F, Aishan H, Zhiqi Z, Weiming L (2015) MiR-193b regulates early chondrogenesis by inhibiting the TGF-beta2 signaling pathway. FEBS Lett 589:1040–1047
Zhen Y, Jie H, Zhen-Ming H (2015) MicroRNA expression profiles in human adipose-derived stem cells during chondrogenic differentiation. Int J Mol Med 35:579–586
Zhang Z, Kang Y, Zhang Z, Zhang H, Duan X, Liu J, Li X, Liao W (2012) Expression of microRNAs during chondrogenesis of human adipose-derived stem cells. Osteoarthritis Cartilage 20:1638–1646
Guping M, Ziji Z, Shu H, Zhiqi Z, Zongkun C, Zhiyu H, Weiming L, Yan K (2018) Exosomes derived from miR-92a-3p overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther 9:247
Mao G, Zhang Z, Huang Z, Chen W, Huang G, Meng F, Zhang Z, Kang Y (2017) MicroRNA-92a-3p regulates the expression of cartilage-specific genes by directly targeting histone deacetylase 2 in chondrogenesis and degradation. Osteoarthritis Cartilage 25:521–532
Changhe H, Ziji Z, Zhiqi Z, Peihui W, Xiaoyi Z, Ming F, Puyi S, Yan K, Weiming L (2015) Presence and function of microRNA-92a in chondrogenic ATDC5 and adipose-derived mesenchymal stem cells. Mol Med Rep 12:4877–4886
Joshua M, Delaine KC, Przemek AG, Prabhu M, Jack FM, Sophia IS, Francesca S, Joshua MH, Susmita S, Roger JH, Kevin DC (2018) Exosomal microRNA-21-5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circ Res 122:933–944
Shi-Cong T, Ting Y, Yue-Lei Z, Wen-Jing Y, Shang-Chun G, Chang-Qing Z (2017) Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7:180–195
Caroline AS, Wayne SR, Kevin WE (2012) NIH image to imageJ: 25 years of image analysis. Nat Methods 9:671–675
Guangju Z, Jules D, Proton R (2015) TGF-β signal transduction pathways and osteoarthritis. Rheumatol Int 35:1283–1292
Erfan AE, Ming L, Patricia EH, Jules D, Glynn M, Andrew F, Roger G, Proton R, Guangju Z (2015) Overexpression of MMP13 in human osteoarthritic cartilage is associated with the SMAD-independent TGF-β signalling pathway. Arthritis Res Ther 17:264
Jie S, Shan L, Di C (2014) TGF-β signaling and the development of osteoarthritis. Bone Res 2:14002
Ude CC, Chen HC, Norhamdan MY, Azizi BM, Aminuddin BS, Ruszymah BHI (2017) The evaluation of cartilage differentiations using transforming growth factor beta3 alone and with combination of bone morphogenetic protein-6 on adult stem cells. Cell Tissue Banking 18:355–367
Zhi-Gao Y, Ruo-Fu T, Yi-Ying Q, Wei-Ping C, Yan X, Li-Dong W (2020) Restoration of cartilage defects using a super paramagnetic iron oxide-labeled adipose-derived mesenchymal stem cell and TGF-β3-loaded bilayer PLGA construct. Regen Med 15:1735–1747
Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL (1997) Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol 139:541–552
Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP (2008) The impact of microRNAs on protein output. Nature 455:64–71
Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N (2008) Widespread changes in protein synthesis induced by microRNAs. Nature 455:58–63
Derynck R, Zhang Y, Feng XH (1998) Transcriptional activators of TGF-β Responses: SMADs. Cell 95:737–740
Joan M, Joan S, David W (2005) SMAD transcription factors. Genes Dev 19:2783–2810
Joan M (2012) TGFβ signalling in context. Nat Rev Mol Cell Biol 13:616–620
Young-Kook K, Boseon K, Kim VN (2016) Re-evaluation of the roles of DROSHA, Export in 5, and DICER in microRNA biogenesis. Proc Natl Acad Sci USA 113:E1881–E1889
Acknowledgements
This work is supported by Japan Society for the Promotion of Science KAKENHI [grant numbers #19K22700, #21H03136]. P0 Human ADSCs were kindly provided by a previous laboratory member Dr. Inaki (Miyagi National Hospital). P0 mouse ADSCs were kindly provided by Dr. IGARASHI (the department of Sensory and Motor System Medicine, the University of Tokyo). P0 Human OA chondrocytes were kindly provided by Dr. Saito (the department of orthopedic surgery and spinal surgery, the University of Tokyo Hospital).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. CZ prepared materials, performed the majority of the experiments described in this manuscript, and participated in the analysis of the histological data. AH and KH supervised the studies, provided technical support, and directed the interpretations of the result. Also, the first draft of the manuscript was written by CZ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Atsuhiko Hikita held an endowed chair supported by FUJISOFT INCORPORATED (until 31 October 2020) and an endowed chair supported by CPC corporation, Kyowa Co., Ltd., Kanto Chemical Co., Inc., and Nichirei Corporation (from 1 July, 2021, to 30 June, 2022), and is affiliated with the social cooperation program of Kohjin Bio Co., Ltd. (since 1 July, 2022). All other authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Zheng, C., Hoshi, K. & Hikita, A. miR-92a-3p-inspired shRNA exhibits pro-chondrogenic and chondrocyte protective effects in osteoarthritis treatment through targeting SMAD6/7. J Bone Miner Metab 42, 1–16 (2024). https://doi.org/10.1007/s00774-023-01474-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-023-01474-3