Abstract
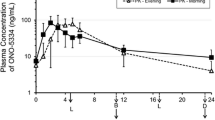
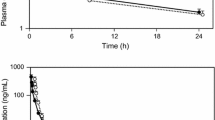
A sustained-release tablet (SRT) of ONO-5334 was compared to the immediate-release tablet (IRT) dose, which demonstrated effects on bone mineral density (BMD) comparable to those of therapy with alendronate. The single-dose phase was a randomized, partial single-blind, crossover study where 50-, 100-, and 300-mg SRTs and 300-mg IRTs were administered to nine post-menopausal women. The multiple-dose phase was a randomized, double-blind, placebo-controlled, parallel-group study where 100- and 300-mg SRTs, or placebo were administered to 24 women. After a single administration of a 300-mg SRT, mean C max was 3.3-fold lower, mean AUCinf was 0.83-fold lower and mean C 24h was 5.4-fold higher compared to the 300-mg IRT. Repeated SRT dosing did not significantly affect PK, although C 24h increased slightly. After a single ONO-5334 dose, serum CTX-I was suppressed by ~50 % within 1 h, reaching maximum suppression 6 h post-dose. Greater suppression was maintained longer by the 300-mg SRT vs. the 300-mg IRT. Second morning void and cumulative urine CTX-I showed clear dose–response effects at/over 24 h for SRT, with maximum suppression occurring at/over 24 h (except 50- and 300-mg cumulative urine). Repeated dosing suggested greater suppression of urine CTX-I. Compared with the IRT, the SRT showed reduced C max, greater C 24h, and slightly reduced AUCinf dose for dose. The SRT showed clear dose–response suppression on bone resorption and greater efficacy dose for dose vs. the IRT.





Similar content being viewed by others
References
Berti PJ, Storer AC (1995) Alignment/phylogeny of the papain superfamily of cysteine proteases. J Mol Biol 246:273–283
Nishi Y, Atley L, Eyre DE, Edelson JG, Superti-Furga A, Yasuda T, Desnick RJ, Gelb BD (1999) Determination of bone markers in pycnodysostosis: effects of cathepsin K deficiency on bone matrix degradation. J Bone Miner Res 14:1902–1908
Gelb BD, Shi GP, Chapman HA, Desnick RJ (1996) Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 273:1236–1238
Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, Moritz JD, Schu P, von Figura K (1998) Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci USA 95:13453–13458
Gowen M, Lazner F, Dodds R, Kapadia R, Feild J, Tavaria M, Bertoncello I, Drake F, Zavarselk S, Tellis I, Hertzog P, Debouck C, Kola I (1999) Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res 14:1654–1663
Data on File. Inhibitory effect of ONO-5334 on human recombinant cathepsin K (E05QA012). Ono Pharmaceutical Co., Ltd.
Data on File. Inhibitory effect of ONO-5334 on 6 cystein proteases (E05QA025). Ono Pharmaceutical Co., Ltd.
Data on File. An Investigation of Ono’s Compound (ONO-5334) on 9 Enzyme and 3 Receptor Binding Assays (1056356). Ono Pharmaceutical Co., Ltd.
Yamada H, Mori H, Nakanishi Y, Kunishige A, Nishikawa S, Tanaka M, Shiroya (2009) Orally active cathepsin K inhibitor, ONO-5334, potently improved bone mineral density not only in trabecular bone but also in cortical bone in ovariectomized cynomolgus monkeys. Presented at American Society for Bone and Mineral Research, abstract A09002146.
Nagase S, Ohyama M, Hashimoto Y, Small M, Kuwayama T, Deacon S (2012) Pharmacodynamic effects on biochemical markers of bone turnover and pharmacokinetics of the cathepsin K inhibitor, ONO-5334, in an ascending multiple dose Phase I study. J Clin Pharm 52:306–318
Eastell R, Nagase S, Ohyama M, Small M, Sawyer J, Boonen S, Spector T, Kuwayama T, Deacon S (2011) Safety and efficacy of the cathepsin K inhibitor ONO-5334 in postmenopausal osteoporosis: the OCEAN study. J Bone Miner Res 26:1303–1312
Eastell R, Nagase S, Small M, Boonen S, Spector T, Ohyama M, Kuwayama T, Deacon S (2013) Effect of ONO-5334 on bone mineral density and biochemical markers of bone turnover in postmenopausal osteoporosis: 2-year results from the OCEAN study. J Bone Miner Res. doi:10.1002/jbmr.2047
Engelke K, Nagase S, Fuerst T, Eastell R, Genant H, Small M, Kuwayama T, and Deacon S (2012) Effects of the cathepsin K inhibitor, ONO-5334, on BMD as measured by 3D QCT in the hip and the spine after 2 years of treatment. Presented at IOF-ECCEO12 European Congress on Osteoporosis and Osteoarthritis, Bordeaux, France
Ochi Y, Yamada H, Mori H, Nakanishi Y, Nishikawa S, Kayasuga R, Kawada N, Kunishige A, Hashimoto Y, Tanaka M, Sugitani M, Kawabata K (2011) Effects of ONO-5334, a novel orally-active inhibitor of cathepsin K, on bone metabolism. Bone 49:1351–1356
Bjarnason NH, Henriksen EEG, Alexandersen P, Christgau S, Henriksen DB, Christiansen C (2002) Mechanism of circadian variation in bone resorption. Bone 30:307–313
Schlemmer A, Hassager C, Jensen SB, Christiansen C (1992) Marked diurnal variation in urinary excretion of pyridinium cross-links in premenopausal women. J Clin Endocrinol Metab 74:476–480
Small M, Dijk D-J, Eastell R, Greenwood A, Sharpe J, Yuba M, Yamada H, Deacon S (2012) Quantification of the circadian modulation of the bone resorption marker CTX-I in serum and urine under controlled in-patient conditions. J Bone Miner Res 27(Suppl 1). http://www.asbmr.org/Meetings/AnnualMeeting/Abstracts12.aspx. Accessed 9 Sept 2013
Acknowledgments
This study was sponsored by Ono Pharmaceutical Co., Ltd. We would like to thank Dr. Gillian Pover for medical support provided to this study, as well as Dr. med. Kathrin Reseski (Principal Investigator) and the staff at PAREXEL International GmbH for their help with this study.
Conflict of interest
All authors are employed by Ono Pharmaceutical Co., Ltd. (Japan) or its European office, ONO PHARMA UK LTD.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nagase, S., Ohyama, M., Hashimoto, Y. et al. Bone turnover markers and pharmacokinetics of a new sustained-release formulation of the cathepsin K inhibitor, ONO-5334, in healthy post-menopausal women. J Bone Miner Metab 33, 93–100 (2015). https://doi.org/10.1007/s00774-013-0558-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-013-0558-2