Summary.
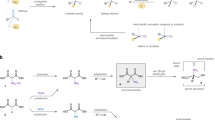
Asymmetric synthesis of all four stereoisomers of 6-methylpipecolic acids with high enantiomeric purity via iterative AD reaction, starting from 1,6-heptadiene, has been described.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received March 25, 2002 Accepted June 15, 2002 Published online January 30, 2003
Authors' address: Hiroki Takahata, Faculty of Pharmaceutical Sciences, Tohoku Pharmaceutical University, Sendai 981-8558, Japan, E-mail: takahata@tohoku-pharm.ac.jp
RID="*"
ID="*" The stereo-configurations of 8 and 9 in Fig. 3 and 12 in Fig. 4 show only major isomers.
RID="**"
ID="**" The absolute configurations of 11 and 14
Rights and permissions
About this article
Cite this article
Takahata, H., Shimizu, M. Asymmetric synthesis of all stereoisomers of 6-methylpipecolic acids. Amino Acids 24, 267–272 (2003). https://doi.org/10.1007/s00726-002-0411-8
Issue Date:
DOI: https://doi.org/10.1007/s00726-002-0411-8