Abstract
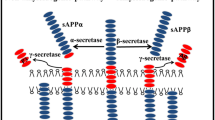
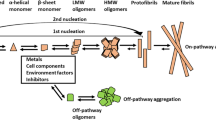
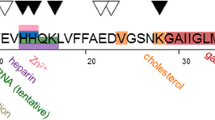
The aggregation of amyloid β (Aβ) peptide is important in Alzheimer’s disease. Shorter Aβ fragments may reduce Aβ’s cytotoxicity and are used in diagnostics. The aggregation of Aβ16 is controversial; Liu et al. (J. Neurosci. Res. 75:162–171, 2004) and Liao et al. (FEBS Lett. 581:1161–1165, 2007) find that Aβ16 does not aggregate and reduces Aβ’s cytotoxicity, Du et al. (J. Alzheimer’s Dis. 27:401–413, 2011) reports that Aβ16 aggregates and that Aβ16 oligomers are toxic to cells. Here the aggregation potential of two shorter fragments, Aβ15 and Aβ16, and their influence on Aβ40 is measured by electron paramagnetic resonance (EPR) spectroscopy and the ThioT fluorescence assay (ThioT). Continuous-wave, 9 GHz EPR measurements and ThioT results reveal that neither Aβ15 nor Aβ16 aggregate by themselves and that they do not affect Aβ40 aggregation.






Similar content being viewed by others
References
K. Chopra, S. Misra, A. Kuhad, Expert Opin. Ther. Targets 15, 535–555 (2011)
R. Jakob-Roetne, H. Jacobsen, Angew. Chem. Int. Ed. Engl. 48, 3030–3059 (2009)
F. Panza, V. Solfrizzi, V. Frisardi, C. Capurso, A. D’Introno, A.M. Colacicco, G. Vendemiale, A. Capurso, B.P. Imbimbo, Drugs Aging 26, 537–555 (2009)
D.J. Selkoe, Ann. Intern. Med. 140, 627–638 (2004)
D.J. Selkoe, Neuron 6, 487–498 (1991)
E. Portelius, G. Brinkmalm, A. Tran, U. Andreasson, H. Zetterberg, A. Westman-Brinkmalm, K. Blennow, A. Ohrfelt, Exp. Neurol. 223, 351–358 (2010)
E. Portelius, N. Mattsson, U. Andreasson, K. Blennow, H. Zetterberg, Curr. Pharm. Des. 17, 2594–2602 (2011)
A. Awasthi, Y. Matsunaga, T. Yamada, Exp. Neurol. 196, 282–289 (2005)
E. Portelius, H. Zetterberg, U. Andreasson, G. Brinkmalm, N. Andreasen, A. Wallin, A. Westman-Brinkmalm, K. Blennow, Neurosci. Lett. 409, 215–219 (2006)
C. Soto, G.P. Saborio, B. Permanne, Acta Neurol. Scand. Suppl. 176, 90–95 (2000)
Y. Matsunaga, A. Fujii, A. Awasthi, J. Yokotani, T. Takakura, T. Yamada, Regul. Pept. 120, 227–236 (2004)
J.F. Leverone, E.T. Spooner, H.K. Lehman, J.D. Clements, C.A. Lemere, Vaccine 21, 2197–2206 (2003)
H. Li, J. Zou, Z. Yao, J. Yu, H. Wang, J. Xu, J. Neuroimmunol. 219, 8–16 (2010)
R. Liu, C. McAllister, Y. Lyubchenko, M.R. Sierks, J. Neurosci. Res. 75, 162–171 (2004)
M.Q. Liao, Y.J. Tzeng, L.Y. Chang, H.B. Huang, T.H. Lin, C.L. Chyan, Y.C. Chen, FEBS Lett. 581, 1161–1165 (2007)
X.T. Du, L. Wang, Y.J. Wang, M. Andreasen, D.W. Zhan, Y. Feng, M. Li, M. Zhao, D. Otzen, D. Xue, Y. Yang, R.T. Liu, J. Alzheimer’s Dis. 27, 401–413 (2011)
A.N. Istrate, P.O. Tsvetkov, A.B. Mantsyzov, A.A. Kulikova, S.A. Kozin, A.A. Makarov, V.I. Polshakov, Biophys. J. 102, 136–143 (2012)
P.O. Tsvetkov, A.A. Kulikova, A.V. Golovin, Y.V. Tkachev, A.I. Archakov, S.A. Kozin, A.A. Makarov, Biophys. J. 99, L84–L86 (2010)
S. Zirah, S.A. Kozin, A.K. Mazur, A. Blond, M. Cheminant, I. Segalas-Milazzo, P. Debey, S. Rebuffat, J. Biol. Chem. 281, 2151–2161 (2006)
I. Sepkhanova, M. Drescher, N.J. Meeuwenoord, R.W.A.L. Limpens, R.I. Koning, D.V. Filippov, M. Huber, Appl. Magn. Reson. 36, 209–222 (2009)
M. Margittai, R. Langen, Q. Rev. Biophys. 41, 265–297 (2008)
F. Scarpelli, M. Drescher, T. Rutters-Meijneke, A. Holt, D.T. Rijkers, J.A. Killian, M. Huber, J. Phys. Chem. B 113, 12257–12264 (2009)
D.C. Rambaldi, A. Zattoni, P. Reschiglian, R. Colombo, L.E. De, Anal. Bioanal. Chem. 394, 2145–2149 (2009)
A.L. Cloe, J.P. Orgel, J.R. Sachleben, R. Tycko, S.C. Meredith, Biochemistry 50, 2026–2039 (2011)
S. Stoll, A. Schweiger, J. Magn. Reson. 178, 42–55 (2006)
S. Steigmiller, M. Börsch, P. Gräber, M. Huber, Biochim. Biophys. Acta 1708, 143–153 (2005)
Q.F. Ma, J. Hu, W.H. Wu, H.D. Liu, J.T. Du, Y. Fu, Y.W. Wu, P. Lei, Y.F. Zhao, Y.M. Li, Biopolymers 83, 20–31 (2006)
M.N. Oda, T.M. Forte, R.O. Ryan, J.C. Voss, Nat. Struct. Biol. 10, 455–460 (2003)
H. LeVine III, Protein Sci. 2, 404–410 (1993)
H. Naiki, K. Higuchi, M. Hosokawa, T. Takeda, Anal. Biochem. 177, 244–249 (1989)
K. Broersen, F. Rousseau, J. Schymkowitz, Alzheimer’s Res. Ther. 2, 12 (2010)
K. Broersen, W. Jonckheere, J. Rozenski, A. Vandersteen, K. Pauwels, A. Pastore, F. Rousseau, J. Schymkowitz, Protein Eng. Des. Sel. 24, 743–750 (2011)
M.L. Giuffrida, F. Caraci, B. Pignataro, S. Cataldo, B.P. De, V. Bruno, G. Molinaro, G. Pappalardo, A. Messina, A. Palmigiano, D. Garozzo, F. Nicoletti, E. Rizzarelli, A. Copani, J. Neurosci. 29, 10582–10587 (2009)
A. Paivio, J. Jarvet, A. Gräslund, L. Lannfelt, A. Westlind-Danielsson, J. Mol. Biol. 339, 145–159 (2004)
O.N. Antzutkin, Magn. Reson. Chem. 42, 231–246 (2004)
C.S. Atwood, R.C. Scarpa, X. Huang, R.D. Moir, W.D. Jones, D.P. Fairlie, R.E. Tanzi, A.I. Bush, J. Neurochem. 75, 1219–1233 (2000)
C.C. Curtain, F. Ali, I. Volitakis, R.A. Cherny, R.S. Norton, K. Beyreuther, C.J. Barrow, C.L. Masters, A.I. Bush, K.J. Barnham, J. Biol. Chem. 276, 20466–20473 (2001)
P. Dorlet, S. Gambarelli, P. Faller, C. Hureau, Angew. Chem. Int. Ed. Engl. 48, 9273–9276 (2009)
J.A. Duce, A. Tsatsanis, M.A. Cater, S.A. James, E. Robb, K. Wikhe, S.L. Leong, K. Perez, T. Johanssen, M.A. Greenough, H.H. Cho, D. Galatis, R.D. Moir, C.L. Masters, C. McLean, R.E. Tanzi, R. Cappai, K.J. Barnham, G.D. Ciccotosto, J.T. Rogers, A.I. Bush, Cell 142, 857–867 (2010)
E. House, J. Collingwood, A. Khan, O. Korchazkina, G. Berthon, C. Exley, J. Alzheimers Dis. 6, 291–301 (2004)
X. Huang, C.S. Atwood, R.D. Moir, M.A. Hartshorn, R.E. Tanzi, A.I. Bush, J. Biol. Inorg. Chem. 9, 954–960 (2004)
D. Jiang, X. Li, L. Liu, G.B. Yagnik, F. Zhou, J. Phys. Chem. B 114, 4896–4903 (2010)
T. Kowalik-Jankowska, M. Ruta, K. Wisniewska, L. Lankiewicz, J. Inorg. Biochem. 95, 270–282 (2003)
T. Kowalik-Jankowska, M. Ruta, K. Wisniewska, L. Lankiewicz, M. Dyba, J. Inorg. Biochem. 98, 940–950 (2004)
S.A. Kozin, Y.V. Mezentsev, A.A. Kulikova, M.I. Indeykina, A.V. Golovin, A.S. Ivanov, P.O. Tsvetkov, A.A. Makarov, Mol. BioSyst. 7, 1053–1055 (2011)
B.K. Shin, S. Saxena, Biochemistry 47, 9117–9123 (2008)
C.D. Syme, R.C. Nadal, S.E.J. Rigby, J.H. Viles, J. Biol. Chem. 279, 18169–18177 (2004)
P.O. Tsvetkov, I.A. Popov, E.N. Nikolaev, A.I. Archakov, A.A. Makarov, S.A. Kozin, ChemBioChem 9, 1564–1567 (2008)
H. Yu, J. Ren, X. Qu, ChemBioChem 9, 879–882 (2008)
Acknowledgments
We acknowledge Edgar Groenen for the constant support and fruitful discussions. This work is part of the research program of the ‘Stichting voor Fundamenteel Onderzoek der Materie (FOM)’, which is financially supported by the “Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO)”, Grant (03BMP03). Financial support from NWO is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shabestari, M., Plug, T., Motazacker, M.M. et al. The Aggregation Potential of the 1–15- and 1–16-Fragments of the Amyloid β Peptide and Their Influence on the Aggregation of Aβ40. Appl Magn Reson 44, 1167–1179 (2013). https://doi.org/10.1007/s00723-013-0469-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-013-0469-3