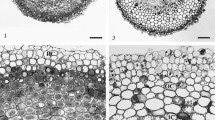
Abstract
K, P, Cl, and Ca are distributed in tissue-specific patterns in Zea mays seedlings. These elements were mapped and analyzed using a relatively simple semi-quantitative technique, i.e., fast freezing, followed by freeze fracturing, then freeze drying, and finally scanning electron microscopy/energy-dispersive spectroscopy (SEM/EDS). In the radicle, endogenously derived (i.e., from seed) K and P transition from being homogenous in the apical meristem to tissue-specific in older regions. At 3 mm from the radicle apex, K concentration is approximately 40 mM in mid-cortex and decreases by approximately 50% at 15 mm. From 3 to 55 mm, P concentration in pericycle is approximately twice that found in adjacent regions. Ca is not detectable in younger portions of the radicle by SEM/EDS, but in older regions, it is present at 13 mM in mid-cortex. K concentration values of entire radicles analyzed with inductively coupled plasma optical emission spectrometry (ICP-OES) exceeded the SEM/EDS values. For Ca, the reverse was true. But, SEM/EDS analysis did not include several vascular tissues that contained high concentrations of K and low concentrations of Ca. The inception of lateral root primordia was accompanied by a localized decrease in Ca in cortical regions that were centrifugal to the primordium tip. A region of O-rich cells in endosperm was identified centripetal to the aleurone. These results indicate that (1) outer, mid-, and inner cortical regions, as well as the adjacent tissues, have distinct ion accumulation properties, and (2) ions are concentrated in some radicle tissues prior to development of Casparian strips.











Similar content being viewed by others
Change history
02 March 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00709-021-01630-4
Abbreviations
- EOC:
-
Epidermis/outer cortical cell layers
- MC:
-
Middle cortex layer
- EPV:
-
Endodermis, pericycle, vascular parenchyma
- Pe:
-
Pericycle
- EPe:
-
Endodermis/pericycle
- MX:
-
Metaxylem
- SEM/EDS:
-
Scanning electron microscopy/energy-dispersive microscopy
- ICP-OES:
-
Inductively coupled plasma optical emission spectrometry
References
Cheah ZX, Kopittke PM, Harper SM, O’Hare TJ, Wang P, Paterson DJ, de Jonge MD, Bell MJ (2018) In situ analyses of inorganic nutrient distribution in sweetcorn and maize kernels using synchrotron-based X-ray fluorescence microscopy. Ann Bot 123:543–556
Chen A, Hansen TH, Olsen LI, Palmgren M, husten S, Schjoerring JK, Persson DP (2020) Towards single-cell ionomics: a novel micro-scaled method for multi-element analysis of nanogram-sized biological samples. Plant Methods 16:31. https://doi.org/10.1186/s13007-020-00566-9
Clarke KJ, McCully ME, Miki NK (1979) A developmental study of the epidermis of young roots of Zea mays L. Protoplasma 98:283–309
Clarkson DT (1984) Calcium transport between tissues and its distribution in the plant. Plant Cell Environ 7:449–456
De Smet I, Vannneste S, Inze D, Beekman T (2006) Lateral root initiation of the birth of anew meristem. Plant Mol Biol 60:871–887
Dembinsky D, Woll K, Saleem M, Liu Y, Fu Y, Borsuk LA, Lamkemyer T, Fladerer C, Madlung J, Barbazuk B, Nordheim A, Bettleton D, Schnable PS, Hochholdinger F (2007) Transcriptomic and proteomic analysis of pericycle cells of the maize primary root. Plant Physiol 145:575–588
Dreyer I, Gomez-Porras JL, Riedelsberger J (2017) The potassium battery: a mobile energy source for transport processes in plant vascular tissues. New Phytol 216:1049–1053
Enstone DE, Peterson CA (2005) Suberin lamella development in maize seedling roots grown in aerated and stagnant conditions. Plant Cell Environ 28:444–455
Erickson RO, Sax KB (1956) Rates of cell division and cell elongation in the growth of the primary root of Zea mays. Proc Am Philos Soc 100:499–514
Frensch J, Hsiao TC, Steudle E (1996) Water and solute transport along developing maize roots. Planta 198:348–355
Geisler-Lee J, Gallie DR (2005) Aleurone cell identity is suppressed following connation in maize kernels. Plant Physiol 139:204–212
Imran I, Garbe-Schonberg D, Neumann G, Boelt B, Muhling KH (2017) Zinc distribution and localization in primed maize seed and its translocation during early seedling development. Environ Exp Bot 143:91–98
Ishikawa H, Hasenstein KH, Evans ML (1991) Computer-based video digitizer analysis of surface extension in maize roots - kinetics of growth-rate changes during gravitropism. Planta 183:381–390
Iwai T, Takahashi M, Oda K, Terada Y, Yoshida KT (2012) Dynamic changes in the distribution of minerals in relation to phytic acid accumulation during rice seed development. Plant Physiol 160:2007–2014
Jones GW, Gorham J 2002. Intra- and inter-cellular compartmentation of ions. In: Lauchli and Luttge (eds) Salinity: Environment-Plants-Molecules. pp. 159–180
Kaneko M, Itoh H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M (2002) The α-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol 128:1264–1270
Kinzel H (1989) Calcium in the vacuoles and cell walls of plant tissue. Flora 182:99–125
Kochian LV, Lucas WJ (1983) Potassium transport in corn roots II. The significance of the root periphery. Plant Physiol 73:208–215
Läuchli A, James RA, Huang CX, McCully M, Munns R (2008) Cell-specific localization of Na+ in roots of durum wheat and possible control points for salt exclusion. Plant Cell Environ 31:1365–1378
Lott JNA, Ockenden I, Raboy V, Batten GD (2000) Phytic acid and phosphorus in crop seeds and fruits: a global estimate. Seed Sci Res 10:11–33
Marcon C, Malik WA, Walley JW, Shen Z, Paschold A, Smith LG, Piepho H-P, Briggs SP, Hochholdinger F (2015) A high-resolution tissue-specific proteome and phosphoproteome atlas of maize primary roots reveals functional gradients along the root axes. Plant Physiol 168:233–246
McCully MC, Canny MJ, Steveninck RFM (1987) Accumulation of potassium by differentiation metaxylem elements of maize roots. Physiol Plant 69:73–80
Møller IS, Gilliham JD, Mayo GM, Roy SJ, Coates JC, Haseloff J, Tester T (2009) Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis
Moreno-Risueno MA, Van Norman JM, Moreno A, Jingyuan Z, Ahnert SE, Benfey PN (2010) Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 329:1306–1311
Nadeem M, Mollier A, Morel C, Shahid M, Aslam M, Zia-ur-Rehaman M, Wahid MA, Pellerin S (2013) Maize seedling phosphorus nutrition: Allocation of remobilized seed phosphorus reserves and external phosphorus uptake to seedling roots and shoots during early growth stages. Plant Soil 371:327–328
Nadeem M, Mollier A, Morel C, Vives A, Pellerin S (2012) Maize endogenous seed phosphorus remobilization is not influenced by exogenous phosphorus availability during germination and early growth stages. Plant Soil 357:13–24
Persson DP, Chen A, Aarts MGM, Salt DE, Schjoerring HS (2016) Multi-element bioimaging of Arabidopsis thaliana roots. Plant Physiol 172:835–847
Pesacreta TC (2015) F-actin distribution in root primary tissues of several seed plant species. Am J Bot 102:1422–1433
Pesacreta TC, Hasenstein KH (2018) Tissue accumulation patterns and concentrations of potassium, phosphorus, and carboxyfluorescein translocated from pine seed to the root. Planta 248:393–407
Pesacreta TC, Purpera MA (2014) Light microscopy survey of extant gymnosperm root protophloem, and comparison with basal angiosperms. Botany 92:388–401
Peterson CA, Emanuel ME, Humphreys GB (1981) Pathway of movement of apoplastic fluorescent dye tracers through the endodermis at the site of secondary root formation in corn (Zea mays) and broad bean (Vicia faba). Can J Bot 59:618–625
Pourcel L, Routaboul JM, Kerhoas L, Caboche M, Lepiniec L, Debeaujon I (2005) TRANSPARENT TESTA10 encodes a Laccase-like enzyme involved in oxidative polymerization of flavonoids in Arabidopsis seed coat. Plant Cell 17:2966–2980
Pritchard J, Fricke W, Tomos D (1996) Turgor-regulation during extension growth and osmotic stress of maize roots. An example of single-cell mapping. Plant Soil 187:11–21
Pritchard J, Williams G, Wyn Jones RG, Tomos AD (1989) Radial turgor pressure profiles in growing and mature zones of wheat roots—a modification of the pressure probe. J Exp Bot 40:567–571
Ragel P, Raddatz N, Leidi EO, Quintero FJ, Pardo JM (2019) Regulation of K+ nutrition in plants. Front Plant Sci 10:281–228
Ricachenevsky FK, de Araujo Junior AT, Fett JP, Sperotto RA (2018) You shall not pass: Root vacuoles as a symplastic checkpoint for metal translocation to shoots and possible application to grain nutritional quality. Front Plant Sci 9:412. https://doi.org/10.3389/fpls.2018.0412
Salt DE, Baxter I, Lahner B (2008) Ionomics and the study of the plant ionome. Annu Rev Plant Biol 59:709–733
Torres-Martinez HH, Rodriguez-Alonso G, Shishkova S, Dubrovsky JG (2019) Lateral foot primordium morphogenesis in angiosperms. Front Plant Sci 10:206. https://doi.org/10.3389/fpls.2019.0206
Van Norman JM, Xuan W, Beekckman T, Benfey PN (2013) To branch or not to branch:the role of pre-patterning in lateral root formation. Development 140:4301–4310
Walker L, Boddington C, Jenkins DJ, Wang Y, Gronlund JT, Hulsmans J, Kumar S, Patel D, Moore JD, Carter A, Samavedam S, Bonom G, Hersh DS, COruzzi G, Burroughs NJ, Gifford M (2017) Changes in gene expression in space and time orchestrate environmentally mediated shaping of root architecture. Plant Cell 29:2393–2412
Walker DJ, Leigh RA, Miller AJ (1996) Potassium homeostasis in vacuolate plant cells. Proc Natl Acad Sci U S A 93:10510–10514
Walter A, Feil R, Schurr U (2003) Expansion dynamics, metabolite composition and substance transfer of the primary root growth zone of Zea mays L. grown in different external nutrient availabilities. Plant Cell Environ 26:1451–1466
White PJ (2001) The pathways of calcium movement to the xylem. J Exp Bot 52:891–899
Woll K, Borsuk LA, Stransky H, Nettleton D, Schnable PS, Hochholdinger F (2005) Isolation, characterization and pericycle-specific transcriptome analyses of the novel maize lateral and seminal root initiation mutant rum11. Plant Physiol 139:1255–1267
Yu P, Baldauf JA, Lithio A, Marcon C, Nettleton D, Li C, Hochholdinger F (2016) Root type- specific reprogramming of maize pericycle transcriptomes by local high nitrate results in disparate lateral root branching patterns. Plant Physiol 170:1783–1798
Zhang NG, Hasenstein KH (1999) Initiation and elongation of lateral roots in Lactuca sativa. Int J Plant Sci 160:511–519
Zhong WJ, Kaiser W, Kohler J, Bauer-Ruckdeschel H, Komor E (1998) Phloem loading of inorganic cations and anions by the seedling of Ricinus communis L. J Plant Physiol 152:328–335
Author information
Authors and Affiliations
Contributions
The authors shared research and manuscript development.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Handling Editor: Alexander Schulz
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pesacreta, T.C., Acharya, A. & Hasenstein, K.H. Endogenous nutrients are concentrated in specific tissues in the Zea mays seedling. Protoplasma 258, 863–878 (2021). https://doi.org/10.1007/s00709-021-01606-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-021-01606-4