Abstract
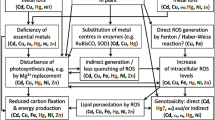
Levels of arsenic (As), chromium (Cr), and copper (Cu) are increasing in the soils worldwide. Such contaminants cause toxicity in the plant systems which adversely affects growth and productivity. The objective of the present investigation was to elucidate individual and combined effects of As, Cr, and Cu (100 μM each) stress in metal hyper-accumulator plant Indian mustard (Brassica juncea L.), exposed for a week. The highest accumulation was in the roots and in decreasing order viz. Cu > As > Cr. The magnitude of oxidative stress was maximal in combined stress, followed by As, Cr, and then Cu stress. Glutathione in conjunction with glutathione reductase, glutathione peroxidase, and glutathione S-transferase increased in all set of stress treatments, notably when exposed to Cr alone. In addition, the level of sulfur-rich compounds like cysteine, phytochelatins, and non-protein thiols increased under each stress indicating efficient coupling of the enzyme system and sulfur-containing compounds during stress conditions. The highest tolerance or growth index of plants was recorded for Cu. Protein profiling of leaf tissues showed modulation of protein patterns in each stress. Mediator of RNA polymerase II transcription subunit 1 isoform X1, RuBisCO (large subunit), and ribosomal protein S3 proteins were more abundant under Cr and Cu stress. Zinc finger A20/AN1 domain-containing stress-associated protein 5–like protein was more abundant under Cu stress. HSP (15.7 kDa) and autophagy protein 5–like were in higher abundance under As and combined stress. Our results suggest that Indian mustard has a differential mode of defense against a particular stressor at the level of protein expression profile.










Similar content being viewed by others
References
Ahmad J, Arif M, Bhajan R, Taj G (2009) Assessment of genetic diversity and genetic relationships among twenty varieties of Brassica juncea L. using RAPD markers. Int J Biotech Biochem 5(1):85–92
Ahmad J, Khan S, Khan GT (2012) Comparative assessment of genetic diversity among twenty varieties of Brassica juncea [L.] Czern & Coss using RAPD and ISSR markers. South Asian J Expt Bio 2(4):166–176
Ahmad J, Bashir H, Bagheri R, Baig A, Al-Huqail A, Ibrahim MM, Qureshi MI (2017) Drought and salinity induced changes in ecophysiology and proteomic profile of Parthenium hysterophorus. PLoS One 12(9):e0185118
Ahmad J, Bagheri R, Bashir H, Baig M, Al-Huqail A, Ibrahim MM, Qureshi MI (2018a) Organ-specific phytochemical profiling and antioxidant analysis of Parthenium hysterophorus L. Biomed Res Int 2018:ID9535232
Ahmad J, Baig MA, Ali AA, Al-Huqail AA, Ibrahim MM, Qureshi MI (2018b) Differential antioxidative and biochemical responses to aluminium stress in Brassica juncea cultivars. Horticulture Env Biotech 59(5):615–627
Ahmad J, Ali AA, Baig MA, Iqbal M, Haq I, Qureshi MI (2019a) Role of phytochelatins in cadmium stress tolerance in plants. In: cadmium toxicity and tolerance in plants. Acad Press, pp 185–212
Ahmad J, Baig MA, Ali AA, Qureshi MI (2019b) Proteomics of cadmium tolerance in plants. In: Cadmium Tolerance in Plants. Acad Press, pp 143–175
Aleamotua M, McCurdy DW, Collings DA (2019) Phi thickenings in roots: novel secondary wall structures responsive to biotic and abiotic stresses. J Exp Bot 39:538–548
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chemother 2019:ID 6730305
Anderson ME, Powrie F, Puri RN, Meister A (1985) Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys 224
Bagheri R, Ahmad J, Bashir H, Iqbal M, Qureshi MI (2017) Changes in rubisco, cysteine-rich proteins and antioxidant system of spinach (Spinacia oleracea L.) due to sulphur deficiency, cadmium stress and their combination. Protoplasma 254(2):1031–1043
Baig MA, Ahmad J, Bagheri R, Ali AA, Al-Huqail A, Ibrahim MM, Qureshi MI (2018) Proteomic and ecophysiological responses of soybean (Glycine max L.) root nodules to Pb and Hg stress. BMC Plant Biol 18(1):283
Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG (2004) Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 25:1327–1333
Cui J-L, Zhao Y-P, Lu Y-J, Chan T-S, Zhang L-L, Tsang DCW, Li X-D (2019) Distribution and speciation of copper in rice (Oryza sativa L.) from mining-impacted paddy soil: Implications for copper uptake mechanisms. Environ Intl 126:717–726
do Nascimento JL, de Almeida AAF, Barroso J, Mangabeira PA, Ahnert D, Sousa AG, Baligar VC (2018) Physiological, ultrastructural, biochemical and molecular responses of young cocoa plants to the toxicity of Cr (III) in soil. Ecotoxicol Environ Saf 159:272–283
Doderer A, Kokkelink I, Van der Veen S, Valk BE, Schram AW, Douma AC (1992) Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim Biophys Acta 1120(1):97–104
Du J, Guo S, Sun J, Shu S (2018) Proteomic and physiological analyses reveal the role of exogenous spermidine on cucumber roots in response to Ca(NO3)2 stress. Plant Mol Biol 97(1–2):1–21
Elia AC, Galarini R, Taticchi MI, Dorr AJM, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurusmelas under mercury exposure. Ecotoxicol Environ Saf 55:162–167
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Franić M, Galić V (2019) As, cd, Cr, Cu, Hg: Physiological implications and toxicity in plants. In: Plant Metallomics and Functional Omics, pp 209–251
Gaitonde MA (1967) Spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem 104:627–633
Gao M, Yang Y, Song Z (2019) Effects of graphene oxide on cadmium uptake and photosynthesis performance in wheat seedlings. Ecotoxicol Environ Saf 173:165–173
Gautam A, Dubey RS (2018) Metal toxicity in plants: induction of oxidative stress, antioxidative defense system, metabolic alterations and phytoremediation. In: Hemantrajan (ed) Molecular physiology of abiotic stresses in plant productivity. Scientific Publishers, Jodhpur, pp 256–290
Gonzaga MIS, da Silva PSO, de Jesus Santos JC, de Oliveira Junior LFG (2019) Biochar increases plant water use efficiency and biomass production while reducing Cu concentration in Brassica juncea L. in a Cu-contaminated soil. Ecotoxicol Environ Saf 183:109557
Gratão PL, Alves LR, Lima LW (2019) Heavy metal toxicity and plant productivity: role of metal scavengers. In: Plant-Metal Interactions, pp 49–60
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Hasanuzzaman M, Hossain MS, Bhuyan MB, Al Mahmud J, Nahar K, Fujita M (2018) The role of sulfur in plant abiotic stress tolerance: molecular interactions and defense mechanisms. In: Plant Nutrients and Abiotic Stress Tolerance, pp 221–252
Hasanuzzaman M, Alam MM, Nahar K, Mohsin SM, Bhuyan MB, Parvin K, Fujita M (2019) Silicon-induced antioxidant defense and methylglyoxal detoxification works coordinately in alleviating nickel toxicity in Oryza sativa L. Ecotoxicology 28(3):261–276
Heath R, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circ Calif Agr Expt Sta 347
Howe G, Merchant S (1992) Heavy metal activated synthesis of peptides in Chlymodomonas reinhartii. Plant Physiol:127–136
Huang Y, Zhu Z, Wu X, Liu Z, Zou J, Chen Y, Cui J (2019) Lower cadmium accumulation and higher antioxidative capacity in edible parts of Brassica campestris L. seedlings applied with glutathione under cadmium toxicity. Environ Sci Pollut Res 26(13):13235–13245
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the Head of Bacteriophage T4. Nature 227(5259):680–685
Javed MT, Tanwir K, Akram MS, Shahid M, Niazi NK, Lindberg S (2019) Phytoremediation of cadmium-polluted water/sediment by aquatic macrophytes: role of plant-induced pH changes. In: cadmium toxicity and tolerance in plants. Acad Press, pp 495–529
Li Z, Song Z, Yan Z, Hao Q, Song A, Liu L, Yang S, Xia S, Liang Y (2018) Silicon enhancement of estimated plant biomass carbon accumulation under abiotic and biotic stresses. A meta-analysis. Agron Sustain Dev 38(3):26
Lu Y, Wang QF, Li J, Xiong J, Zhou LN, He SL, Liu H (2019) Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Sci Rep 9(1):7397
Marchiol L, Assolari S, Sacco P, Zerbi G (2004) Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ Pollut 132(1):21–27
Maresca V, Sorbo S, Keramat B, Basile A (2017) Effects of triacontanol on ascorbate-glutathione cycle in Brassica napus L. exposed to cadmium-induced oxidative stress. Ecotoxicol Environ Saf 144:268–274
Muszyńska E, Labudda M (2019) Dual role of metallic trace elements in stress biology-from negative to beneficial impact on plants. Int J Mol Sci 20(13):3117
Niazi NK, Bibi I, Fatimah A, Shahid M, Javed MT, Wang H, Ok YS, Bashir S, Murtaza B, Saqib ZA, Shakoor MB (2017) Phosphate-assisted phytoremediation of arsenic by Brassica napus and Brassica juncea: morphological and physiological response. Int J Phytoremediat 19(7):670–678
Nouri J, Lorestani B, Yousefi N, Khorasani N, Hasani AH, Seif F, Cheraghi M (2011) Phytoremediation potential of native plants grown in the vicinity of Ahangaran lead–zinc mine (Hamedan, Iran). Environ Earth Sci 62(3):639–644
Pan G, Zhang H, Liu P, Xiao Z, Li X, Liu W (2019) Effects of manganese stress on phenology and biomass allocation in Xanthium strumarium from metalliferous and non-metalliferous sites. Ecotoxicol Environ Saf 172:308–316
Paul M, Goswami C, Mukherjee M, Roychowdhury T (2019) Phyto-remedial detoxification of arsenic by Pistia stratiotes and assessment of its anti-oxidative enzymatic changes. Bioremed J:1–10
Qadir S, Qureshi MI, Javed S, Abdin MZ (2004) Genotypic variation in phytoremediation potential of Brassica juncea cultivars exposed to Cd stress. Plant Sci 167:1171–1181
Qureshi MI, D’Amici GM, Fagioni M, Rinalducci S, Zolla L (2010) Iron stabilizes thylakoid protein–pigment complexes in Indian mustard during Cd-phytoremediation as revealed by BN-SDS-PAGE and ESI-MS/MS. J Plant Physiol 167(10):761–770
Rahman Z, Singh VP (2019) The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ Monit Assess 191(7):419
Rizwan M, Ali S, ur Rehman MZ, Rinklebe J, Tsang DC, Bashir A, Maqbool A, Tack FMG, Ok YS (2018) Cadmium phytoremediation potential of Brassica crop species: a review. Sci Total Environ 631:1175–1191
Safa M, O'Carroll D, Mansouri N, Robinson B, Curline G (2020) Investigating arsenic impact of ACC treated timbers in compost production (a case study in Christchurch, New Zealand). Environ Pollut 114218
Scarpeci TE, Zanor MI, Carrillo N, Mueller-Roeber B, Valle EM (2008) Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: a focus on rapidly induced genes. Plant Mol Biol 66:361–378
Shen J, Su Y, Zhou C, Zhang F, Zhou H, Liu X, Yin X, Wu D, Yuan X (2019) A putative rice l-cysteine desulfhydrase encodes a true l-cysteine synthase that regulates plant cadmium tolerance. Plant Growth Regul:1–10
Šiukšta R, Bondzinskaitė S, Kleizaitė V, Žvingila D, Taraškevičius R, Mockeliūnas L, Čėsnienė T (2019) Response of Tradescantia plants to oxidative stress induced by heavy metal pollution of soils from industrial areas. Environ Sci Pollut Res 26(1):44–61
Su D, Jiang R, and Li H (2018). The potential of oilseed rape and Thlaspi caerulescens for phytoremediation of cadmium-contaminated soil. In twenty years of research and development on soil pollution and remediation in China 349-363
Thakur M, Udayashankar AC (2019) Lipoxygenases and their function in plant innate mechanism. In: Jogaiah S, Abdelrahman M (eds) Bioactive molecules in plant defense. Springer, Cham
Wang J, Yu Q, Xiong H, Wang J, Chen S, Yang Z, Dai S (2016) Proteomic insight into the response of Arabidopsis chloroplasts to darkness. PLoS One 11(5):e0154235
Watson BS, Asirvatham VS, Wang L, Sumner LW (2003) Mapping the proteome of barrel medic (Medicago truncatula). Plant Physiol 131(3):1104–1123
Xia J, Sinelnikov IV, Han B, Wishart DS (2015) MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res 43:W251–W257
Zhu Y, Wang B, Phillips J, Zhang ZN, Du H, Xu T, Wang Z, Wang L, Deng X (2015) Global transcriptome analysis reveals acclimation-primed processes involved in the acquisition of desiccation tolerance in Boea hygrometrica. Plant Cell Physiol 56(7):1429–1441
Acknowledgments
Metal analysis in plant samples facilitated by Central Pollution Control Board, New Delhi, India, is appreciatively acknowledged.
Funding
This work is supported by King Saud University, Riyadh, Saudi Arabia through researchers supporting Project Number RSP-2020/186.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests
Additional information
Handling Editor: Néstor Carrillo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1711 kb)
Rights and permissions
About this article
Cite this article
Ahmad, J., Qamar, S., Nida et al. Differential impact of some metal(loid)s on oxidative stress, antioxidant system, sulfur compounds, and protein profile of Indian mustard (Brassica juncea L.). Protoplasma 257, 1667–1683 (2020). https://doi.org/10.1007/s00709-020-01535-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01535-8