Abstract
An improved environmentally benign procedure for the synthesis of substituted 2-thio-benzothia(oxa)zoles, 2-thiobenzimidazoles, and 1,3-oxazolopyridine-2-thiols by cyclization of 2-aminophenols, 2-aminothiophenols, 1,2-phenylenediamines, or 2-amino-3-hydroxypyridines with potassium O-ethyldithiocarbonate in PEG 400 or glycerol under directed microwave irradiation is described. The method can be applied to the synthesis of a variety of derivatives.
Graphical abstract


Similar content being viewed by others
References
Singh MS, Singh P, Singh S (2007) Indian J Chem 46B:1666
Tsushima M, Kano Y, Umemura E, Iwamatsu K, Tamura A, Shibahara S (1998) Bioorg Med Chem 6:1641
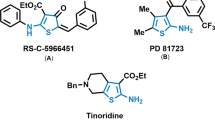
Easmon J, Heinisch G, Hofmann J, Langer T, Grunicke HH, Fink J, Pürstinger G (1997) Eur J Med Chem 32:397
Wood WW (2002) US Patent 6,448,262
Ramadas K, Janarthanan N (1999) Synth Commun 29:1003
Huang W, Yang GF (2006) Bioorg Med Chem 14:8280
Handte R, Hörlein G, Köcher H, Langelüddeke P (1978) US Patent 4,130,413
Cossey HD, Judd J, Stephens FF (1965) J Chem Soc 954
Bujdáková H, Kuchta T, Sidóová E, Gvozdjaková A (1993) FEMS Microbiol Lett 112:329
Mortimer CG, Wells G, Crochard JP, Stone EL, Bradshaw TD, Stevens MFG, Westwell AD (2006) J Med Chem 49:179
Deligeorgiev T, Kaloyanova S, Lesev N, Vaquero JJ (2010) Ultrason Sonochem 17:783
Furstenberg A, Julliard MD, Deligeorgiev T, Gadjev N, Vasilev A, Vauthey E (2006) J Am Chem Soc 128:7661
Kaloyanova S, Trusova V, Gorbenko G, Deligeorgiev T (2010) J Photochem Photobiol A 217:147
Zhivotova TS, Gazaliev AM, Fazylov SD, Aitpaeva ZK, Turdybekov DM (2006) Russ J Org Chem 42:448
Aliev NA, Tashkhodzhaev B, Levkovich MG, Abdullaev ND, Kartstev VG (1997) Khim Geterotsikl Soedin 11:1545
Davidkov K, Simov D (1981) Khim Geterotsikl Soedin 5:608
Thakuria H, Das G (2008) Arkivoc xv:321
Doise M, Blondeau D, Sliwa H (1992) Synth Commun 22:2891
Wang M-L, Liu B-L (2007) J Chin Inst Chem Eng 38:161
Chen J, Spear SK, Huddleston JG, Rogers RD (2005) Green Chem 7:64
Wolfson A, Dlugy C, Shotland Y (2007) Environ Chem Lett 5:67
Knochel P (1999) Modern solvents in organic synthesis. Springer, Berlin
Gu Y, Barrault J, Jerrome F (2008) Adv Synth Catal 350:2007
Wolfson A, Litvak G, Dlugy C, Shotland Y, Tavor D (2009) Ind Crops Prod 30:78
Mac M, Baran W, Uchacz T, Baran B, Suder M, Leśniewski S (2007) J Photochem Photobiol A 192:188
Harizi A, Romdhane A, Mighri Z (2000) Tetrahedron Lett 41:5833
Handte R, Sander J, Tammer TUS (1984) US Patent 4,442,294
Kalcheva-Batchvarova VB, Boteva PC, Antonova AT, Petrov OI, Mincheva ZP, Caignard DH, Renard P, Bizot-Espiard JG (1998) WO9825913
Pappne Behr A, Kapui Z, Aranyi P, Batori S, Bartanr Vodor V, Varga M, Mikus E, Urban-Szabo K, Vargane Szeredi J, Szabo T, Susan E, Kovacs M (2007) WO2007034253
Cemiani A, Passerini R (1954) Ann Chim 44:3
Katz L, Cohen MS (1954) J Org Chem 19:758
Valdez J, Cedillo R, Herna′ndez-Campos A, Yepez L L, Hernandez-Luis F, Navarrete-Vazquez G, Tapia A, Cortes R, Hernandezc M, Castilloa R (2002) Bioorg Med Chem Lett 12:2221
Carato P, Moussavi Z, Sabaouni A, Lebegue N, Berthelot P, Yous S (2006) Tetrahedron 62:9054
Moussavi Z, Lesieur D, Lespagnol C, Sauzieres J, Olivier P (1989) Eur J Med Chem 24:55
Acknowledgments
The authors acknowledge financial support (in part) from the Spanish Ministerio de Ciencia e Innovación (project CTQ2008-04313/BQU) and from the Bulgarian National Science Fund (grant DO1-873).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Julio Álvarez-Builla on the occasion of his 65th birthday.
Rights and permissions
About this article
Cite this article
Deligeorgiev, T.G., Kaloyanova, S.S., Lesev, N.Y. et al. An environmentally benign procedure for the synthesis of substituted 2-thiobenzothiazoles, 2-thiobenzoxazoles, 2-thiobenzimidazoles, and 1,3-oxazolopyridine-2-thiols. Monatsh Chem 142, 895–899 (2011). https://doi.org/10.1007/s00706-011-0551-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0551-1