Abstract
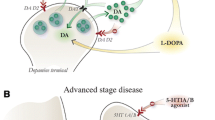
The MPTP monkey model of Parkinson’s disease (PD) has allowed huge advances regarding the understanding of the pathological mechanisms of PD and l-DOPA-induced adverse effects. Among the main findings were the imbalance between the efferent striatal pathways in opposite directions between the hypokinetic and hyperkinetic states of PD. In both normal and parkinsonian monkeys, the combination of behavioral and anatomical studies has allowed the deciphering of the cortico-basal ganglia circuits involved in both movement and behavioral disorders. A major breakthrough has then been made regarding the hypothesis of the involvement of serotonergic fibers in the conversion of l-DOPA to dopamine when dopaminergic neurons are dying and to release it, in an uncontrolled manner, as serotonergic neurons are deprived from the machinery required for buffering dopamine from the synaptic cleft. The crucial involvement of serotonergic fibers underlying l-DOPA-induced dyskinesia (LID) has been demonstrated in both rodent and monkey models of PD, in which dyskinesia induced by l-DOPA is abolished following lesion of the serotonergic system. Moreover, the role of serotonergic fibers goes well beyond dyskinesia, as lesioning of such serotonergic fibers by MDMA in the monkey also decreased other l-DOPA-induced adverse effects such as impulsive compulsive behaviors and visual hallucinations. The same pathological mechanism, i.e., an imbalance between serotonin and dopamine terminals may, therefore, favor l-DOPA-induced adverse effects according to the basal ganglia territory it inhabits. Further non-human primate studies will be needed to demonstrate the role of such a pathological mechanism in both movement and behavioral disorders driven by l-DOPA therapy but also to determine the causal link between serotonin lesions and the expression of non-motor symptoms like apathy, depression and anxiety, frequently observed in PD patients.




Similar content being viewed by others
References
Ahlskog JE, Muenter MD (2001) Frequency of l-DOPA-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16(3):448–458
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375
Alexander GE (1994) Basal ganglia-thalamocortical circuits: their role in control of movements. J Clin Neurophysiol 11(4):420–431
Baizer JS, Desimone R, Ungerleider LG (1993) Comparison of subcortical connections of inferior temporal and posterior parietal cortex in monkeys. Vis Neurosci 10:59–72
Ballanger B, Strafella AP, van Eimeren T, Zurowski M, Rusjan PM, Houle S, Fox SH (2010) Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol 67:416–421
Ballanger B, Beaudoin-Gobert M, Neumane S, Epinat J, Metereau E, Duperrier S, Broussolle E, Thobois S, Bonnefoi F, Tourvielle C, Lavenne F, Costes N, Lebars D, Zimmer L, Sgambato-Faure V, Tremblay L (2016) Imaging dopamine and serotonin systems on MPTP monkeys: a longitudinal pet investigation of compensatory mechanisms. J Neurosci 36(5):1577–1589
Bastide MF, Meissner WG, Picconi B, Fasano S, Fernagut PO, Feyder M, Francardo V, Alcacer C, Ding Y, Brambilla R, Fisone G, Jon Stoessl A, Bourdenx M, Engeln M, Navailles S, De Deurwaerdère P, Ko WK, Simola N, Morelli M, Groc L, Rodriguez MC, Gurevich EV, Quik M, Morari M, Mellone M, Gardoni F, Tronci E, Guehl D, Tison F, Crossman AR, Kang UJ, Steece-Collier K, Fox S, Carta M, Angela Cenci M, Bézard E (2015) Pathophysiology of l-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol 132:96–168
Battaglia G, De Souza EB (1989) Pharmacologic profile of amphetamine derivatives at various brain recognition sites: selective effects on serotonergic systems. NIDA Res Monogr 94:240–258
Beaudoin-Gobert M, Epinat J, Metereau E, Duperrier S, Neumane S, Ballanger B, Lavenne F, Liger F, Tourvielle C, Bonnefoi F, Costes N, Bars D, Broussolle E, Thobois S, Tremblay L, Sgambato-Faure V (2015) Behavioural impact of a double dopaminergic and serotonergic lesion in the non-human primate. Brain 138:2632–2647
Bédard PJ, Di Paolo T, Falardeau P, Boucher R (1986) Chronic treatment with l-DOPA, but not bromocriptine induces dyskinesia in MPTP-parkinsonian mon-keys. Correlation with [3H]spiperone binding. Brain Res 379(2):294–299
Bezard E, Brotchie JM, Gross CE (2001) Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci 2(8):577–588
Bézard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P (2003) Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat Med 9(6):762–767
Blesa J, Phani S, Jackson-Lewis V, Przedborski S (2012) Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol 2012:845618
Boyce S, Rupniak NM, Steventon MJ, Iversen SD (1990) Characterisation of dyskinesias induced by l-dopa in MPTP-treated squirrel monkeys. Psychopharmacology 102(1):21–27
Burbaud P, Bonnet B, Guehl D, Lagueny A, Bioulac B (1998) Movement disorders induced by gamma-aminobutyric agonist and antagonist injections into the internal globus pallidus and substantia nigra pars reticulata of the monkey. Brain Res 780(1):102–107
Burns LH, Pakzaban P, Deacon TW, Brownell AL, Tatter SB, Jenkins BG, Isacson O (1995) Selective putaminal excitotoxic lesions in non-human primates model the movement disorder of Huntington disease. Neuroscience 64(4):1007–1017
Carta M, Tronci E (2014) Serotonin system implication in l-DOPA-induced dyskinesia: from animal models to clinical investigations. Front Neurol 5:78
Carta M, Carlsson T, Kirik D, Björklund A (2007) Dopamine released from 5-HT terminals is the cause of l-DOPA-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833
Cenci MA (2014) Presynaptic mechanisms of l-DOPA induced dyskinesia: the findings, the debate, and the therapeutic implications. Front Neurol 5:242
Clarke CE, Sambrook MA, Mitchell IJ, Crossman AR (1987) Levodopa-induced dyskinesia and response fluctuations in primates rendered parkinsonian with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). J Neurol Sci 78(3):273–280
Crossman AR (1987) Primate models of dyskinesia: the experimental approach to the study of basal ganglia-related involuntary movement disorders. Neuroscience 21(1):1–40
Crossman AR, Sambrook MA, Jackson A (1980) Experimental hemiballismus in the baboon produced by injection of a gamma-aminobutyric acid antagonist into the basal ganglia. Neurosci Lett 20(3):369–372
Crossman AR, Sambrook MA, Jackson A (1984) Experimental hemichorea/hemiballismus in the monkey. Studies on the intracerebral site of action in a drug induced dyskinesia. Brain 107(Pt 2):579–596
Crossman AR, Mitchell IJ, Sambrook MA, Jackson A (1988) Chorea and myoclonus in the monkey induced by gamma-aminobutyric acid antagonism in the lentiform complex. The site of drug action and a hypothesis for the neural mechanisms of chorea. Brain 111(Pt 5):1211–1233
De Souza EB, Battaglia G, Insel TR (1990) Neurotoxic effect of MDMA on brain serotonin neurons: evidence from neurochemical and radioligand binding studies. Ann N Y Acad Sci 600:682–697 (discussion 697–688)
de Win MM, Jager G, Booij J, Reneman L, Schilt T, Lavini C, Olabarriaga SD, den Heeten GJ, van den Brink W (2008) Sustained effects of ecstasy on the human brain: a prospective neuroimaging study in novel users. Brain 131:2936–2945
DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13(7):281–285
Di Monte DA, McCormack A, Petzinger G, Janson AM, Quik M, Langston WJ (2000) Relationship among nigrostriatal denervation, parkinsonism, and dyskinesias in the MPTP primate model. Mov Disord 15:459–466
Diederich NJ, Goetz CG, Stebbins GT (2005) Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: focused review and a new integrative model. Mov Disord 20:130–140
Elbaz A, Damier P (2004) Epidemiology of Parkinson’s disease. La letter du Neurologue 8(1):13–15 (French)
Eskow KL, Dupre KB, Barnum CJ, Dickinson SO, Park JY, Bishop C (2009) The role of the dorsal raphe nucleus in the development, expression, and treatment of l-dopa-induced dyskinesia in hemiparkinsonian rats. Synapse 63:610–620
Filion M, Tremblay L, Bédard PJ (1991) Effects of dopamine agonists on the spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res 547(1):152–161
Fox SH, Brotchie JM (2010) The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present, and future. Prog Brain Res 184:133–157 (Review)
Fox SH, Chuang R, Brotchie JM (2008) Parkinson’s disease—opportunities for novel therapeutics to reduce the problems of levodopa therapy. Prog Brain Res 172:479–494
Fox SH, Visanji N, Reyes G, Huot P, Gomez-Ramirez J, Johnston T, Brotchie JM (2010) Neuropsychiatric behaviors in the MPTP marmoset model of Parkinson’s disease. Can J Neurol Sci 37:86–95
Fox SH, Johnston TH, Li Q, Brotchie J, Bezard E (2012) A critique of available scales and presentation of the non-human primate dyskinesia rating scale. Mov Disord 2:1373–1378
François C, Grabli D, McCairn K, Jan C, Karachi C, Hirsch EC, Féger J, Tremblay L (2004) Behavioural disorders induced by external globus pallidus dysfunction in primates II. Anatomical study. Brain 127(Pt 9):2055–2070
Galineau L, Kas A, Worbe Y, Chaigneau M, Herard AS, Guillermier M, Delzescaux T, Féger J, Hantraye P, Tremblay L (2017) Cortical areas involved in behavioral expression of external pallidum dysfunctions: a PET imaging study in non-human primates. Neuroimage 146:1025–1037
Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, Sibley DR (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250(4986):1429–1432
Gottwald MD, Aminoff MJ (2011) Therapies for dopaminergic-induced dyskinesias in Parkinson disease. Ann Neurol 69:919–927
Gough B, Ali SF, Slikker W Jr, Holson RR (1991) Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on monoamines in rat caudate. Pharmacol Biochem Behav 39:619–623
Grabli D, McCairn K, Hirsch EC, Agid Y, Féger J, François C, Tremblay L (2004) Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain 127(Pt 9):2039–2054
Graybiel AM (2005) The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol 15(6):638–644
Guehl D, Pessiglione M, François C, Yelnik J, Hirsch EC, Féger J, Tremblay L (2003) Tremor-related activity of neurons in the ‘motor’ thalamus: changes in firing rate and pattern in the MPTP vervet model of parkinsonism. Eur J Neurosci 17(11):2388–2400
Guridi J, González-Redondo R, Obeso JA (2012) Clinical features, pathophysiology, and treatment of levodopa-induced dyskinesias in Parkinson’s disease. Parkinsons Dis 2012:943159
Hantraye P, Riche D, Maziere M, Isacson O (1990) A primate model of Huntington’s disease: behavioral and anatomical studies of unilateral excitotoxic lesions of the caudate-putamen in the baboon. Exp Neurol 108(2):91–104
Hatzidimitriou G, McCann UD, Ricaurte GA (1999) Altered serotonin innervation patterns in the forebrain of monkeys treated with (±)3,4-methylenedioxymethamphetamine 7 years previously: factors influencing abnormal recovery. J Neurosci 19:5096–5107
Huot P, Johnston TH, Darr T, Hazrati LN, Visanji NP, Pires D, Brotchie JM, Fox SH (2010) Increased 5-HT2A receptors in the temporal cortex of parkinsonian patients with visual hallucinations. Mov Disord 25:1399–1408
Huot P, Fox SH, Brotchie JM (2011) The serotonergic system in Parkinson’s disease. Prog Neurobiol 95:163–212
Imbert C, Bezard E, Guitraud S, Boraud T, Gross CE (2000) Comparison of eight clinical rating scales used for the assessment of MPTP-induced parkinsonism in the Macaque monkey. J Neurosci Methods 96:71–76
Insel TR, Battaglia G, Johannessen JN, Marra S, De Souza EB (1989) 3,4-Methylenedioxymethamphetamine (“ecstasy”) selectively destroys brain serotonin terminals in rhesus monkeys. J Pharmacol Exp Ther 249:713–720
Iravani MM, Syed E, Jackson MJ, Johnston LC, Smith LA, Jenner P (2005) A modified MPTP treatment regime produces reproducible partial nigrostriatal lesions in common marmosets. Eur J Neurosci 21(4):841–854
Jan C, Pessiglione M, Tremblay L, Tandé D, Hirsch EC, François C (2003) Quantitative analysis of dopaminergic loss in relation to functional territories in MPTP-treated monkeys. Eur J Neurosci 18(7):2082–2086
Jenner P (2003) The MPTP-treated primate as a model of motor complications in PD: primate model of motor complications. Neurology 61((6) Suppl. 3):S4–S11
Karachi C, Grabli D, Baup N, Mounayar S, Tandé D, François C, Hirsch EC (2009) Dysfunction of the subthalamic nucleus induces behavioral and movement disorders in monkeys. Mov Disord 24(8):1183–1192
Karuppagounder SS, Bhattacharya D, Ahuja M, Suppiramaniam V, Deruiter J, Clark R, Dhanasekaran M (2014) Elucidating the neurotoxic effects of MDMA and its analogs. Life Sci 101:37–42
Kim HF, Hikosaka O (2015) Parallel basal ganglia circuits for voluntary and automatic behaviour to reach rewards. Brain 138(Pt 7):1776–1800
Manson AJ, Schrag A (2006) Levodopa-induced dyskinesias, the clinical problem: clinical features, incidence, risk factors, management and impact on quality of life. In: Bezard E (ed) Recent breakthroughs in Basal Ganglia Research. Nova Science Publishers Inc., New York, pp 369–380
Matsumura M, Tremblay L, Richard H, Filion M (1995) Activity of pallidal neurons in the monkey during dyskinesia induced by injection of bicuculline in the external pallidum. Neuroscience 65:59–70
Meissner W, Prunier C, Guilloteau D, Chalon S, Gross CE, Bezard E (2003) Time-course of nigrostriatal degeneration in a progressive MPTP-lesioned macaque model of Parkinson’s disease. Mol Neurobiol 28(3):209–218
Météreau E, Beaudoin-Gobert M, Duperrier S, Thobois S, Tremblay L, Sgambato-Faure V (2017) Diffusion tensor imaging marks dopaminergic and serotonergic lesions in the Parkinsonian monkey. Mov Disord. https://doi.org/10.1002/mds.27201 (Epub ahead of print)
Middleton FA, Strick PL (1996) The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci USA 93:8683–8687
Middleton FA, Strick PL (2000) Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn 42(2):183–200 (Review)
Mink JW (1996) The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50(4):381–425 (Review)
Moratalla R, Quinn B, DeLanney LE, Irwin I, Langston JW, Graybiel AM (1992) Differential vulnerability of primate caudate-putamen and striosome-matrix dopamine systems to the neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci USA 89(9):3859–3863
Morissette M, Di Paolo T (2017) Non-human primate models of PD to test novel therapies. J Neural Transm (Vienna). https://doi.org/10.1007/s00702-017-1722-y
Mounayar S, Boulet S, Tande D, Jan C, Pessiglione M, Hirsch EC, Feger J, Savasta M, Francois C, Tremblay L (2007) A new model to study compensatory mechanisms in MPTP treated monkeys exhibiting recovery. Brain 130:2898–2914
Munoz A, Li Q, Gardoni F, Marcello E, Qin C, Carlsson T, Kirik D, Di Luca M, Bjorklund A, Bezard E, Carta M (2008) Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of l-DOPA-induced dyskinesia. Brain 131:3380–3394
Nambu A (2004) A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res 143:461–466 (Review)
Navailles S, De Deurwaerdère P (2012) Contribution of serotonergic transmission to the motor and cognitive effects of high-frequency stimulation of the subthalamic nucleus or levodopa in Parkinson’s disease. Mol Neurobiol 45:173–185
Obeso JA, Olanow CW, Nutt JG (2000) Levodopa motor complications in Parkinson’s disease. Trends Neurosci 23:S2–S7
Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Arbizu J, Gimenez-Amaya JM (2002) The basal ganglia and disorders of movement: pathophysiological mechanisms. News Physiol Sci 17:51–55
Obeso JA, Rodriguez-Oroz MC, Stamelou M, Bhatia KP, Burn DJ (2014) The expanding universe of disorders of the basal ganglia. Lancet 384(9942):523–531
Olanow CW, Obeso JA, Stocchi F (2006) Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet Neurol 5(8):677–687
Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev 20:128–154
Pessiglione M, Guehl D, Hirsch EC, Féger J, Tremblay L (2004) Disruption of self-organized actions in monkeys with progressive MPTP-induced Parkinsonism. I. Effects of task complexity. Eur J Neurosci 19(2):426–436
Politis M (2014) Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol 10:708–722
Politis M, Niccolini F (2015) Serotonin in Parkinson’s disease. Behav Brain Res 277:136–145
Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, Bjorklund A, Lindvall O, Piccini P (2010) Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci Transl Med 2:38ra46
Politis M, Oertel WH, Wu K, Quinn NP, Pogarell O, Brooks DJ et al (2011) Graft-induced dyskinesias in Parkinson’s disease: high striatal serotonin/dopamine transporter ratio. Mov Disord 26:1997–2003
Politis M, Wu K, Loane C, Brooks DJ, Kiferle L, Turkheimer FE, Bain P, Molloy S, Piccini P et al (2014) Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson’s disease patients. J Clin Investig 124:1340–1349
Potts LF, Wu H, Singh A, Marcilla I, Luquin MR, Papa SM (2014) Modeling Parkinson’s disease in monkeys for translational studies, a critical analysis. Exp Neurol 256:133–143
Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc JL (2003) Limitations of current Parkinson’s disease therapy. Ann Neurol 53(Suppl. 3):S12–S15 (S3–12 discussion)
Ricaurte GA, Forno LS, Wilson MA, DeLanney LE, Irwin I, Molliver ME, Langston JW (1988) (±)3,4-Methylenedioxymethamphetamine selectively damages central serotonergic neurons in nonhuman primates. JAMA 260:51–55
Ricaurte GA, Yuan J, McCann UD (2000) (±)3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology 42:5–10
Roussakis AA, Politis M, Towey D, Piccini P (2016) Serotonin-to-dopamine transporter ratios in Parkinson disease: relevance for dyskinesias. Neurology 86(12):1152–1158
Rudnick G, Wall SC (1992) The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci USA 89:1817–1821
Rylander D, Parent M, O’Sullivan SS, Dovero S, Lees AJ, Bézard E et al (2010) Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann Neurol 68:619–628
Saga Y, Richard A, Sgambato-Faure V, Hoshi E, Tobler PN, Tremblay L (2017) Ventral pallidum encodes contextual information and controls aversive behaviors. Cereb Cortex 27(4):2528–2543
Schneider JS (1989) Levodopa-induced dyskinesias in parkinsonian monkeys: relationship to extent of nigrostriatal damage. Pharmacol Biochem Behav 34(1):193–196
Schneider JS, Pope-Coleman A (1995) Cognitive deficits precede motor deficits in a slowly progressing model of parkinsonism in the monkey. Neurodegeneration 4(3):245–255
Sgambato-Faure V, Cenci MA (2012) Glutamatergic mechanisms in the dyskinesias induced by pharmacological dopamine replacement and deep brain stimulation for the treatment of Parkinson’s disease. Prog Neurobiol 96(1):69–86
Sgambato-Faure V, Tremblay L (2017) Dopamine and serotonin modulation of motor and non-motor functions of the non-human primate striato-pallidal circuits in normal and pathological states. J Neural Transm (Vienna). https://doi.org/10.1007/s00702-017-1693-z ((Epub ahead of print) Review)
Sgambato-Faure V, Worbe Y, Epinat J, Feger J, Tremblay L (2016) Cortico-basal ganglia circuits involved in different motivation disorders in non-human primates. Brain Struct Funct 221:345–364
Smith R, Wu K, Hart T, Loane C, Brooks DJ, Björklund A, Odin P, Piccini P, Politis M (2015) The role of pallidal serotonergic function in Parkinson’s disease dyskinesias: a positron emission tomography study. Neurobiol Aging 36(4):1736–1742
Song DD, Haber SN (2000) Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci 20:5102–5114
Stowe RL, Ives NJ, Clarke C, van Hilten J, Ferreira J, Hawker RJ, Shah L, Wheatley K, Gray R (2008) Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev 2:CD006564
Szabo Z, McCann UD, Wilson AA, Scheffel U, Owonikoko T, Mathews WB, Ravert HT, Hilton J, Dannals RF, Ricaurte GA (2002) Comparison of (+)-(11)C-McN5652 and (11)C-DASB as serotonin transporter radioligands under various experimental conditions. J Nucl Med 43:678–692
Tanaka H, Kannari K, Maeda T, Tomiyama M, Suda T, Matsunaga M (1999) Role of serotonergic neurons in l-DOPA-derived extracellular dopamine in the striatum of 6-OHDA-lesioned rats. Neuroreport 10:631–634
Tremblay L, Worbe Y, Thobois S, Sgambato-Faure V, Feger J (2015) Selective dysfunction of basal ganglia subterritories: from movement to behavioral disorders. Mov Disord 30:1155–1170
Visanji NP, Gomez-Ramirez J, Johnston TH, Pires D, Voon V, Brotchie JM, Fox SH (2006) Pharmacological characterization of psychosis-like behavior in the MPTP-lesioned nonhuman primate model of Parkinson’s disease. Mov Disord 21:1879–1891
Voon V, Napier TC, Frank MJ, Sgambato-Faure V, Grace AA, Rodriguez-Oroz M, Obeso J, Bezard E, Fernagut PO (2017) Impulse control disorders and levodopa-induced dyskinesias in Parkinson’s disease: an update. Lancet Neurol 16(3):238–250
Williams DR, Lees AJ (2005) Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: a retrospective autopsy study. Lancet Neurol 4:605–610
Worbe Y, Baup N, Grabli N, Chaigneau M, Mounayar S, McCairn K, Féger J, Tremblay L (2009) Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb Cortex 19(8):1844–1856
Worbe Y, Epinat J, Féger J, Tremblay L (2011) Discontinuous long-train stimulation in the anterior striatum in monkeys induces abnormal behavioral states. Cereb Cortex 21(12):2733–2741
Acknowledgements
Dr V. Sgambato is supported by INSERM (Institut National de la Santé et de la Recherche Médicale) and Dr L Tremblay by CNRS (Centre National de Recherche Scientifique).
Funding
This work was supported by Grants from Fondation de France (Grant numbers 201234497, 00060911 and 00016818) and Labex Cortex (ANR-11-LABX-0042).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Sgambato, V., Tremblay, L. Pathophysiology of dyskinesia and behavioral disorders in non-human primates: the role of serotonergic fibers. J Neural Transm 125, 1145–1156 (2018). https://doi.org/10.1007/s00702-018-1871-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-018-1871-7



