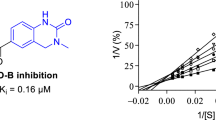
Abstract
A series of 1-[2-((5-methyl/chloro)-2-benzoxazolinone-3-yl)acetyl]-3,5-diaryl-4,5-dihydro-1H-pyrazole derivatives were prepared by reacting 2-((5-methyl/chloro)-2-benzoxazolinone-3-yl)acetylhydrazine with appropriate chalcones. The chemical structures of all compounds were confirmed by elemental analyses, IR, 1H NMR and ESI–MS. All the compounds were investigated for their ability to selectively inhibit monoamine oxidase (MAO) by in vitro tests. MAO activities of the compounds were compared with moclobemide and selegiline and all the compounds were found to inhibit human MAO-A selectively. The inhibition profile was found to be competitive and reversible for all compounds by in vitro tests. Among the compounds examined, compounds 5ae, 5af and 5ag were more selective than moclobemide, with respect to the K i values experimentally found. In addition, the compound 5bg showed MAO-A inhibitor activity as well as moclobemide. A series of experimentally tested compounds (5ae–5ch) were docked computationally to the active site of the MAO-A and MAO-B isoenzyme. The AUTODOCK 4.01 program was employed to perform automated molecular docking.


Similar content being viewed by others
References
Anderson MC, Hasan F, McCrodden JM, Tipton KF (1993) Monoamine oxidase inhibitors and the cheese effect. Neurochem Res 18:1145–1149
Bilgin AA, Palaska E, Sunal R (1993) Studies on the synthesis and antidepressant activity of Some 1-thiocarbamoyl-3,5-diphenyl-2-pyrazolines. Arzneimittel-Forsch 43:1041–1044
Binda C, Wang J, Pisani L, Caccia C, Carotti A, Salvati P, Edmondson DE, Mattevi A (2007) Structures of human monoamine oxidase B complexes with selective noncovalent inhibitors: safinamide and coumarin analogs. J Med Chem 50:5848–5852
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Çakır B, Dağ Ö, Yıldırım E, Erol K, Şahin MF (2001) Synthesis and anticonvulsant activity of some hydrazones of 2[(3H)-oxobenzoxazolin-3-yl-aceto]hydrazide. J Fac Pharm Gazi 18:99–106
Chen SQ, Zhang YC, Liu FM (2011) Synthesis and spectral characterization of some new thiazolyl-pyrazolines bearing 1,2,4-triazole moiety. Phosphorus Sulfur 186:319–325
Chimenti F, Bolasco A, Manna F, Secci D, Chimenti P et al (2004) Synthesis and selective inhibitory activity of 1-acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives against monoamine oxidase. J Med Chem 47:2071–2074
Chimenti F, Maccioni E, Secci D, Bolasco A, Chimenti P, Granese A et al (2005) Synthesis, molecular modeling studies, and selective inhibitory activity against monoamine oxidase of 1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazole derivatives. J Med Chem 48:7113–7122
Chimenti F, Bolasco A, Manna F, Secci D, Chimenti P, Granese A, Befani O, Turini P, Alcaro S, Ortuso F (2006a) Synthesis and molecular modelling of novel substituted-4,5-dihydro-(1H)-pyrazole derivatives as potent and highly selective monoamine oxidase-A inhibitors. Chem Biol Drug Des 67:206–214
Chimenti F, Bolasco A, Manna F, Secci D, Chimenti P, Granesea A, Befani O, Turini P, Cirilli R et al (2006b) Synthesis, biological evaluation and 3D-QSAR of 1,3,5-trisubstituted-4,5-dihydro-(1H)-pyrazole derivatives as potent and highly selective monoamine oxidase A inhibitors. Curr Med Chem 13:1411–1428
Chimenti F, Fioravanti R, Bolasco A, Manna F, Chimenti P et al (2007) Monoamine oxidase isoform-dependent tautomeric influence in the recognition of 3,5-diaryl pyrazole ınhibitors. J Med Chem 50:425–428
Chimenti F, Fioravanti R, Bolasco A, Manna F, Chimenti P et al (2008a) Synthesis, molecular modeling studies and selective inhibitory activity against MAO of N1-propanoyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives. Eur J Med Chem 43:2262–2267
Chimenti F, Maccioni E, Secci D, Bolasco A, Chimenti P et al (2008b) Synthesis, stereochemical identification, and selective inhibitory activity against human monoamine oxidase-B of 2-methylcyclohexylidene-(4-arylthiazol-2-yl)hydrazones. J Med Chem 51:4874–4880
Chimenti F, Carradori S, Secci D, Bolasco A, Bizzarri B et al (2010) Synthesis and inhibitory activity against human monoamine oxidase of N1-thiocarbamoyl-3,5-di(hetero)aryl-4,5-dihydro-(1H)-pyrazole derivatives. Eur J Med Chem 45:800–804
Close WJ, Tiffany BD, Spielman MA (1949) The analgesic activity of some benzoxazolone derivatives. J Am Chem Soc 71:1265–1268
Dawey W, Tivey DJ (1958) Chalcones and related compounds. Part IV. Addition of hydrogen cyanide to chalcones 242:1230–1236
Dong F, Jian C, Zhenghao F, Kai G, Zuliang L (2008) Synthesis of chalcones via Claisen–Schmidt condensation reaction catalyzed by acyclic acidic ionic liquids. Catal Commun 9:1924–1927
Drukarch B, van Muiswinkel FL (2000) Drug treatment of Parkinson’s disease. Time for phase II. Biochem Pharmacol 59:1023–1031
Gökçe M, Geciken AE, Yıldırım E, Tosun AU (2001) Synthesis and anticonvulsant activity of 5-chloro-2(3H)-benzoxazolinone-3-acetyl-2-(o/p-substituted benzal)hydrazone derivatives. Arzneimittelforschung 58:537–542
Gökhan N, Yeşilada A, Uçar G, Erol K, Bilgin AA (2003) 1-N-Substituted thiocarbamoyl-3-phenyl-5-thienyl-2-pyrazolines: synthesis and evaluation as MAO inhibitors. Arch Pharm Pharm Med Chem 336:362–371
Gökhan-Kelekçi N, Yabanoğlu S, Küpeli E, Salgın U, Özgen Ö et al (2007) A new therapeutic approach in alzheimer disease: some novel pyrazole derivatives as dual MAO-B inhibitors and antiinflammatory analgesics. Bioorg Med Chem 15:5775–5786
Gökhan-Kelekçi N, Koyunoğlu S, Yabanoğlu S, Yelekçi K, Özgen Ö et al (2009) New pyrazoline bearing 4(3H)-quinazolinone inhibitors of monoamine oxidase: synthesis, biological evaluation, and structural determinants of MAO-A and MAO-B selectivity. Bioorg Med Chem 17:675–689
Holla BS, Akberali PM, Shivananda MK (2000) Studies on arylfuran derivatives-Part X. Synthesis and antibacterial properties of arylfuryl-∆ 2-pyrazolines. Il Farmaco 55:256–263
Irie K, Watanabe K (1980) Aldol condensations with metal(II) complex catalysts. Bull Chem Soc Jpn 53:1366–1371
Kubota Y, Ikeya H, Sugi Y, Yamada T, Tatsumi T (2006) Organic-inorganic hybrid catalysts based on ordered porous structures for Michael reaction. J Mol Catal A-Chem 249:181–190
Lipson VV, Desenko SM, Shirobokova MG, Borodina VV, Musatov VI (2005) Chemical reactions of 2-methyl-5,7-diphenyl-6,7-dihydropyrazolo[1,5-a]pyrimidine (New York, NY, United States). Chem Heterocycl Compd 41:492–495
Manna F, Chimenti F, Bolasco A, Secci D, Bizzarri B et al (2002) Inhibition of amine oxidases activity by 1-acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives. Bioorg Med Chem Lett 12:3629–3633
Milcent R, Akhnazarian A, Lensen N (1996) Synthesis of 1-(2-hydroxyphenyl)-2,4-imidazolidinedione derivatives through cyclic transformations of ethyl 2-oxo-3(2H)-benzoxazoleacetate derivatives. J Heterocycl Chem 33:1829–1833
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) Autodock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comp Chem 16:2785–2791
Önkol T, Gökçe M, Tosun AU, Polat S, Serin MS, Tezcan S (2008) Microwave synthesis and antimicrobial evaluation of 5-chloro-2(3H)-benzoxazolinone-3-acetyl-2-(p-substituted benzal)hydrazone and 5-chloro-2(3H)-benzoxazolinone-3-acetyl-2-(p-substituted acetophenone)hydrazone derivatives. Turk J Pharm Sci 5:155–166
Özdemir Z, Kandilci HB, Gümüşel B, Çalış Ü, Bilgin AA (2007) Synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-furyl)-pyrazoline derivatives. Eur J Med Chem 42:373–379
Özdemir Z, Kandilci HB, Gümüşel B, Çalış Ü, Bilgin AA (2008) Synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-thienyl)-pyrazoline derivatives. Arch Pharm Chem Life Sci 341:701–707
Palaska E, Aytemir M, Uzbay İT, Erol D (2001) Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur J Med Chem 36:539–543
Palaska E, Aydın F, Uçar G, Erol D (2008) Synthesis and monoamine oxidase inhibitory activities of 1-thiocarbamoyl-3,5-diphenyl-4,5-dihydro-1H-pyrazole derivatives. Arch Pharm Chem Life Sci 341:209–215
Potts KT, Bhattacharjee D, Kanemasa S (1980) Mesoionic compounds. 52. Attempted synthesis of the anhydro-2-hydroxyoxazolo[2,3b-]oxazolium hydroxide system. J Org Chem 45:4985–4988
Şahin ZS, Salgın-Gökşen U, Gökhan-Kelekçi N, Işık Ş (2011) Synthesis, crystal structures and DFT studies of 1-[2-(5-methyl-2-benzoxazolinone-3-yl)acetyl]-3-phenyl-5-(3,4-dimethoxyphenyl)-4,5-dihydro-1H-pyrazole and 1-[2-(5-chloro-2-benzoxazolinone-3-yl)acetyl]-3-phenyl-5-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole. J Mol Struct 1006:147–158
Salgın-Gökşen U, Gökhan-Kelekçi N, Göktaş Ö, Köysal Y, Kılıç E et al (2007) 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg Med Chem 15:5738–5751
Sarabhai KP, Mathur KBL (1963) Some arylation reactions with diazotized 3,4,5-trimethoxyaniline. Preparation of 3,4,5-trimethoxychalcone and 3,4,5-trimethoxybenzaldehyde. Indian J Chem 1:482–483
Schapira AH (2007) Treatment options in the modern management of Parkinson disease. Arch Neurol 64:1083–1088
Secci D, Bolasco A, Chimenti P, Carradori S (2011) The state of the art of pyrazole derivatives as monoamine oxidase inhibitors and antidepressant/anticonvulsant agents. Curr Med Chem 18:5114–5144
Shekarchi M, Pirali-Hamedani M, Navidpour L, Adib N, Shafiee A (2008) Synthesis, antibacterial and antifungal activities of 3-aryl-5-(pyridin-3-yl)-4,5-dihydropyrazole-1-carbothioamide derivatives. J Iran Chem Soc 5:150–158
Son S-Y, Ma J, Kondou Y, Yoshimura M, Yamashita E, Tsukihara T (2008) Structure of human monoamine oxidase A at 2.2-Å resolution: the control of opening the entry for substrates/inhibitors. P Natl Acad Sci USA 105:5739–5744
Ueno Y, Yadav LDS, Okawara M (1983) Carbon-carbon bond formation via phosphine-initiated cleavage of oxosulphides. Chem Lett 6:831–834
Ünlü S, Erdoğan H, Sunal R (1992) Synthesis of some (2-benzoxazolinones-3-yl)alkonoic acid derivatives and their analgesic properties. Hacettepe University J Faculty of Pharm 12:23–31
Yamada M, Yasuhara H (2004) Clinical pharmacology of MAO inhibitors: safety and future. Neurotoxicology 25:215–221
Yáñez M, Fraiz N, Cano E, Orallo F (2006) Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem Biophys Res Commun 344:688–695
Yelekçi K, Karahan Ö, Toprakci M (2007) Docking of novel reversible monoamine oxidase-B inhibitors: efficient prediction of ligand binding sites and estimation of inhibitors thermodynamic properties. J Neural Transm 114:725–732
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salgın-Gökşen, U., Yabanoğlu-Çiftçi, S., Ercan, A. et al. Evaluation of selective human MAO inhibitory activities of some novel pyrazoline derivatives. J Neural Transm 120, 863–873 (2013). https://doi.org/10.1007/s00702-013-0980-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-013-0980-6