Abstract
Purpose
Recently, the Zurich Pituitary Score (ZPS) has been proposed as a new quantitative preoperative classification scheme for predicting gross total resection (GTR), extent of resection (EOR), and residual tumor volume (RV) in endoscopic pituitary surgery. We evaluated the external validity of the ZPS.
Methods
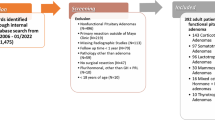
In three reference centers for pituitary surgery, the ZPS was applied and correlated to GTR, EOR, and RV. Furthermore, its inter-rater agreement was assessed.
Results
A total of 485 patients (53% male; age, 53.8 ± 15.7) were included. ZPS grades I, II, III, and IV were observed in 110 (23%), 270 (56%), 64 (13%), and 41 (8%) patients, respectively. GTR was achieved in 358 (74%) cases, with mean EOR of 87.6% ± 20.3% and RV of 1.42 ± 2.80 cm3. With increasing ZPS grade, strongly significant decreasing trends for GTR (I, 92%; II, 77%; III, 67%; IV, 15%; p < 0.001) and EOR (I, 93.8%; II, 89.9%; III, 88.1%; IV, 75.4%; p < 0.001) were found. Similarly, RV increased steadily ([cm3] I, 0.16; II, 0.61; III, 2.01; IV, 3.84; p < 0.001). We observed intraclass correlation coefficients of 0.837 (95% CI, 0.804–0.865) for intercarotid distance and 0.964 (95% CI, 0.956–0.970) for adenoma diameter, and Cohen’s kappa of 0.972 (95% CI, 0.952–0.992) for the ZPS grades.
Conclusions
Application of the ZPS in three external cohorts was successful. The ZPS generalized well in terms of GTR, EOR, and RV; demonstrated excellent inter-rater agreement; and can safely and effectively be applied as a quantitative classification of adenomas with relevance to surgical outcome.




Similar content being viewed by others
References
Amrhein V, Greenland S, McShane B (2019) Scientists rise up against statistical significance. Nature 567(7748):305
Biller BMK, Grossman AB, Stewart PM et al (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93(7):2454–2462
Bossuyt PM, Reitsma JB, Bruns DE et al (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 351:h5527
Bouthillier A, van Loveren HR, Keller JT (1996) Segments of the internal carotid artery: a new classification. Neurosurgery 38(3):425–432 discussion 432-433
Cappabianca P, Cavallo LM, Colao A, de Divitiis E (2002) Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg 97(2):293–298
Cappabianca P, Cavallo LM, de Divitiis E (2004) Endoscopic endonasal transsphenoidal surgery. Neurosurgery 55(4):933–940 discussion 940-941
Cebula H, Kurbanov A, Zimmer LA, Poczos P, Leach JL, De Battista JC, Froelich S, Theodosopoulos PV, Keller JT (2014) Endoscopic, endonasal variability in the anatomy of the internal carotid artery. World Neurosurg 82(6):e759–e764
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20(1):37–46
Dallapiazza RF, Grober Y, Starke RM, Laws ER, Jane JA (2015) Long-term results of endonasal endoscopic transsphenoidal resection of nonfunctioning pituitary macroadenomas. Neurosurgery 76(1):42–52 discussion 52-53
Dehdashti AR, Ganna A, Karabatsou K, Gentili F (2008) Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series. Neurosurgery 62(5):1006–1015 discussion 1015-1017
Dhandapani S, Singh H, Negm HM, Cohen S, Anand VK, Schwartz TH (2016) Cavernous sinus invasion in pituitary adenomas: systematic review and pooled data meta-analysis of radiologic criteria and comparison of endoscopic and microscopic surgery. World Neurosurg 96:36–46
Elhadi AM, Hardesty DA, Zaidi HA, Kalani MYS, Nakaji P, White WL, Preul MC, Little AS (2015) Evaluation of surgical freedom for microscopic and endoscopic transsphenoidal approaches to the sella. Neurosurgery 11(Suppl 2):69–78 discussion 78-79
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES, MacDonald RL, Mayer SA (2006) Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery 59(1):21–27 discussion 21-27
Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, Trainer P, Ghigo E, Ho K, Melmed S (2010) A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab 95(7):3141–3148
Hardy J, Vezina JL (1976) Transsphenoidal neurosurgery of intracranial neoplasm. Adv Neurol 15:261–273
Hughes JD, Koeller K, Rinaldo L, Erickson D, Bancos I, Meyer FB, Atkinson J, Van Gompel JJ (2018) Beyond gross total and subtotal: does volumetric resection matter in nonfunctioning pituitary macroadenomas? World Neurosurg 116:e733–e737
Kanter AS, Dumont AS, Asthagiri AR, Oskouian RJ, Jane JA, Laws ER (2005) The transsphenoidal approach. A historical perspective. Neurosurg Focus 18(4):e6
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4):610–617 discussion 617-618
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Maldaner N, Stienen MN, Bijlenga P et al (2017) Interrater agreement in the radiologic characterization of ruptured intracranial aneurysms based on computed tomography angiography. World Neurosurg 103:876–882.e1
Mattogno PP, D’Alessandris QG, Fraschetti F, Lauretti L (2019) Going beyond scoring systems for cavernous sinus involvement in trans-sphenoidal pituitary surgery. Acta Neurochir 161(5):1033–1034
Mehta GU, Oldfield EH (2012) Prevention of intraoperative cerebrospinal fluid leaks by lumbar cerebrospinal fluid drainage during surgery for pituitary macroadenomas. J Neurosurg 116(6):1299–1303
Meij BP, Lopes M-BS, Ellegala DB, Alden TD, Laws ER (2002) The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J Neurosurg 96(2):195–208
Micko ASG, Wöhrer A, Wolfsberger S, Knosp E (2015) Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J Neurosurg 122(4):803–811
Mooney MA, Hardesty DA, Sheehy JP, Bird R, Chapple K, White WL, Little AS (2016) Interrater and intrarater reliability of the Knosp scale for pituitary adenoma grading. J Neurosurg 126(5):1714–1719
Mooney MA, Hardesty DA, Sheehy JP, Bird CR, Chapple K, White WL, Little AS (2017) Rater reliability of the Hardy classification for pituitary adenomas in the magnetic resonance imaging era. J Neurol Surg Part B Skull Base 78(5):413–418
Mooney MA, Sarris CE, Zhou JJ et al (2019) Proposal and validation of a simple grading scale (TRANSSPHER grade) for predicting gross total resection of nonfunctioning pituitary macroadenomas after transsphenoidal surgery. Oper Neurosurg Hagerstown Md. https://doi.org/10.1093/ons/opy401
Negm HM, Al-Mahfoudh R, Pai M, Singh H, Cohen S, Dhandapani S, Anand VK, Schwartz TH (2017) Reoperative endoscopic endonasal surgery for residual or recurrent pituitary adenomas. J Neurosurg 127(2):397–408. https://doi.org/10.3171/2016.8.JNS152709
Obermeyer Z, Emanuel EJ (2016) Predicting the future - big data, machine learning, and clinical medicine. N Engl J Med 375(13):1216–1219
Perkins NJ, Schisterman EF (2006) The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 163(7):670–675
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Serra C, Regli L (2019) Response to: “No doubt: the invasion of the cavernous sinus is the limiting factor for complete resection in pituitary adenomas”. Acta Neurochir 161(4):719–720
Serra C, Burkhardt J-K, Esposito G, Bozinov O, Pangalu A, Valavanis A, Holzmann D, Schmid C, Regli L (2016) Pituitary surgery and volumetric assessment of extent of resection: a paradigm shift in the use of intraoperative magnetic resonance imaging. Neurosurg Focus 40(3):E17
Serra C, Maldaner N, Muscas G, Staartjes V, Pangalu A, Holzmann D, Soyka M, Schmid C, Regli L (2017) The changing sella: internal carotid artery shift during transsphenoidal pituitary surgery. Pituitary 20(6):654–660. https://doi.org/10.1007/s11102-017-0830-x
Serra C, Staartjes VE, Maldaner N, Muscas G, Akeret K, Holzmann D, Soyka MB, Schmid C, Regli L (2018) Predicting extent of resection in transsphenoidal surgery for pituitary adenoma. Acta Neurochir 160(11):2255–2262
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86(2):420–428
Staartjes VE, Serra C, Muscas G, Maldaner N, Akeret K, van Niftrik CHB, Fierstra J, Holzmann D, Regli L (2018) Utility of deep neural networks in predicting gross-total resection after transsphenoidal surgery for pituitary adenoma: a pilot study. Neurosurg Focus 45(5):E12
Staartjes VE, Serra C, Maldaner N, Muscas G, Tschopp O, Soyka MB, Holzmann D, Regli L (2019) The Zurich Pituitary Score predicts utility of intraoperative high-field magnetic resonance imaging in transsphenoidal pituitary adenoma surgery. Acta Neurochir https://doi.org/10.1007/s00701-019-04018-9
Staartjes VE, Zattra CM, Akeret K, Maldaner N, Muscas G, van Niftrik CHB, Fierstra J, Regli L, Serra C (2019) Neural network–based identification of patients at high risk for intraoperative cerebrospinal fluid leaks in endoscopic pituitary surgery. J Neurosurg 1–7. https://doi.org/10.3171/2019.4.JNS19477
Steinmetz MP, Mroz T (2018) Value of adding predictive clinical decision tools to spine surgery. JAMA Surg https://doi.org/10.1001/jamasurg.2018.0078
Streitberg B, Röhmel J (1986) Exact distributions for permutation and rank tests: an introduction to some recently published algorithms. Stat Softw Newsl 12(1):10–17
Sughrue ME, Chang EF, Gabriel RA, Aghi MK, Blevins LS (2011) Excess mortality for patients with residual disease following resection of pituitary adenomas. Pituitary 14(3):276–283
Sylvester PT, Evans JA, Zipfel GJ et al (2015) Combined high-field intraoperative magnetic resonance imaging and endoscopy increase extent of resection and progression-free survival for pituitary adenomas. Pituitary 18(1):72–85
Wilson CB (1979) Clinical management of pituitary disorders. Neurosurg Manag Large Invasive Pituit Tumors Tindall GT Collins WF Ed Raven Press N Y 335–342
Zoli M, Milanese L, Bonfatti R, Sturiale C, Pasquini E, Frank G, Mazzatenta D (2016) Cavernous sinus invasion by pituitary adenomas: role of endoscopic endonasal surgery. J Neurosurg Sci 60(4):485–494
Acknowledgments
We thank Mr. Nicola Podda for his original artwork (Figure 1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Cantonal Ethics Committee Zürich, KEK St-V-Nr 2015-0142) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pituitaries
Electronic supplementary material
Supplementary methods 1
R Code for the statistical analysis and rendering of the figure. The code was executed in R Version 3.5.2 (The R Foundation for Statistical Computing, Vienna, Austria) on a machine running Windows 10 (Microsoft Corp., Redmond, WA, USA). (R 10 kb)
Supplementary Figure 1
Pooled gross total resection (GTR) rates among the Knosp grades. (JPEG 3651 kb)
Supplementary Table 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Staartjes, V.E., Serra, C., Zoli, M. et al. Multicenter external validation of the Zurich Pituitary Score. Acta Neurochir 162, 1287–1295 (2020). https://doi.org/10.1007/s00701-020-04286-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04286-w