Abstract
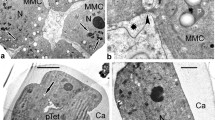
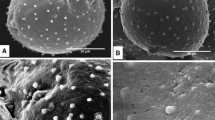
Pollen wall development of Sciadopitys verticillata was observed by transmission electron microscopy. The pollen of S. verticillata is non-saccate and spherical, and the exine consists of the outer thick, sculptured ectexine and the inner lamellated endexine. At the early tetrad stage, the initial ectexine and lamellae of the initial endexine begin to form on the microspore plasma membrane. The ectexine granules gradually swell. Deposition of sporopollenin materials on the ectexine granules then results it their becoming partially connected to each other. Identification of the original small ectexine granules then becomes difficult, and, finally, the ectexine appears as a homogeneous, partially discontinuous layer. The granules of the early ectexine cannot be identified. At maturity, there are four to five endexine lamellae. Recent molecular data have shown that Sciadopitys first branches off from the Cupressaceae plus Taxaceae clade, which is characterized by granular exine. Although the ectexine of Sciadopitys is similar to that of the Cupressaceae during initial development, the morphology of the ectexine is significantly different in the mature pollen. The initial stage of pollen development clearly shows the structural homology of the granular ectexine. Divergence of the exine structure occurs in the later stages.


Similar content being viewed by others
References
Bowe LM, Coat G, de Pamphilis CW (2000) Phylogeny of seed plants based on all three genomic compartments: extant gymnosperms are monophyletic and Gnetales’ closest relatives are conifers. Proc Natl Acad Sci USA 97:4092–4097
Chaw SM, Zharkikh A, Sung HM, Lau TC, Li WH (1997) Molecular phylogeny of extant gymnosperms and seed plant evolution: analysis of nuclear 18S rRNA sequences. Mol Biol Evol 14:56–68
Chaw SM, Parkinson CL, Cheng Y, Vincent TM, Palmer JD (2000) Seed plant phylogeny inferred from all three plant genomes: monophyly of extant gymnosperms and origin of Gnetales from conifers. Proc Natl Acad Sci USA 97:4086–4091
Doyle JA (2009) Evolutionary significance of granular exine structure in the light of phylogenetic analyses. Rev Palaeobot Palynol 156:198–210
Florin R (1922) On the geological history of the Sciadopitineae. Svensk Bot Tidskr 16:260–270
Gadek PA, Alpers DL, Heslewood MM, Quinn CJ (2000) Relationships within Cupressaceae sensu lato: a combined morphological and molecular approach. Am J Bot 87:1044–1057
Gugerli F, Sperisen C, Büchler U, Brunner I, Brodbeck S, Palmer JD, Qiu YL (2001) The evolutionary split of Pinaceae from other conifers: evidence from an intron loss and a multigene phylogeny. Mol Phylogenet Evol 21:167–175
Gullvåg BM (1966) The fine structure of some gymnosperm pollen walls. Grana Palynol 6:435–475
Heslop-Harrison J (1963) An ultrastructural study of pollen wall ontogeny in Silene pendula. Grana Palynol 4:7–24
Karnovsky MJ (1965) A formaldehyde–glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137A–138A
Kurmann MH (1990a) Exine formation in Cunninghamia lanceolata (Taxodiaceae). Rev Palaeobot Palynol 64:175–179
Kurmann MH (1990b) Exine ontogeny in conifers. In: Blackmore S, Knox RB (eds) Microspore: evolution and ontogeny. Academic Press, London, pp 157–172
Kurmann MH (1992) Exine stratification in extant gymnosperms: a review of published transmission electron micrographs. Kew Bull 47:25–39
Kurmann MH, Zavada MS (1994) Pollen morphological diversity in extant and fossil gymnosperms. In: Kurmann MH, Doyle JA (eds) Ultrastructure of fossil spores and pollen. Royal Botanic Garden, Kew, pp 123–137
Lawson AA (1910) The gametophytes and embryo of Sciadopitys verticillata. Ann Bot 24:403–422
Lugardon B (1995) Exine formation in Chamaecyparis lawsoniana (Cupressaceae) and a discussion on pteridophyte exospore and gymnosperm exine ontogeny. Rev Palaeobot Palynol 85:35–51
Médus J, Gajardo R, Woltz P (1989) Exine ultrastructure of Dacrydium fonkii, Saxegothaea conspicua and Stachycarpus andina (Podocarpaceae) from southern South America. Grana 28:19–23
Page CN (1990) Sciadopityaceae. In: Kramer KU, Green PS (eds) The families and genera of vascular plants I. Pteridophytes and gymnosperms. Springer, Berlin, pp 346–348
Peirce AS (1936) Anatomical interrelationships of the Taxodiaceae. Trop Woods 46:1–15
Pettitt JM (1974) The surface coats on spores. Biol J Linn Soc 6:245–257
Quinn CJ, Price RA, Gadek PA (2002) Familial concepts and relationships in the conifers based on rbcL and matK sequence comparisons. Kew Bull 57:513–531
Rai HS, Reeves PA, Peakall R, Olmstead RG, Graham SW (2008) Inference of higher-order conifer relationships from a multi-locus plastid data set. Botany 86:658–669
Rowley JR (1973) Formation of pollen exine bacules and microchannels on a glycocalyx. Grana 13:129–138
Said C (1989) Some characteristics of pollen wall cytochemistry and ultrastructure in Japanese larch (Larix leptolepis Gord.). Sex Plant Reprod 2:77–84
Schmidt M, Schneider-Poetsch HAW (2002) The evolution of gymnosperms redrawn by phytochrome genes: the Gnetatae appear at the base of the gymnosperms. J Mol Evol 54:715–724
Seward AC (1919) Fossil plants, vol 4. Cambridge Univ Press, Cambridge
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastr Res 26:31–43
Stefanovic S, Jager M, Deutsch J, Broutin J, Masselot M (1998) Phylogenetic relationships of conifers inferred from partial 28S rRNA gene sequences. Am J Bot 85:688–697
Surova TD, Kvavadze EV (1988) Sporoderm ultrastructure in some gymnosperms (Metasequoia, Cunninghamia, Sciadopitys). Bot Zhurn (Moscow and Leningrad) 73:34–44
Uehara K, Sahashi N (2000) Pollen wall development in Cryptomeria japonica. Grana 39:267–274
Ueno J (1951) Morphology of pollen of Metaseqoia, Sciadopitys and Taiwania. J Inst Polytechn Osaka City Univ Ser D 2:22–28
Ueno J (1958) Some palynological observations of Pinaceae. J Inst Polytechn Osaka City Univ Ser D 9:163–187
Ueno J (1960) Studies on pollen grains of Gymnospermae. Concluding remarks to relationship between Coniferae. J Inst Polytech Osaka City Univ Ser D 11:109–136
Ueno J (1978) Study of palynology. Kazama Shobo, Tokyo
Van Campo M (1971) Précisions nouvelles sur les structures comparées des pollens de Gymnospermes et d’Angiospermes. C R Acad Sc Paris Ser D 272:2071–2074
Van Campo M, Lugardon B (1973) Structure grenue infratectale de l’ectexine des pollens de quelques Gymnospermes et Angiospermes. Pollen Spores 15:171–187
Xi YZ, Wang FH (1989) Pollen exine ultrastructure of extant Chinese gymnosperms. Cathaya 1:119–142
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uehara, K., Saiki, K. Pollen wall development in Sciadopitys verticillata (Sciadopityaceae). Plant Syst Evol 294, 177–183 (2011). https://doi.org/10.1007/s00606-011-0449-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-011-0449-8