Abstract
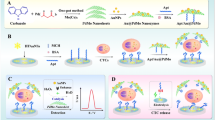
As a real-time fluid biopsy method, the detection of circulating tumor cells (CTCs) provides important information for the early diagnosis, precise treatment, and prognosis of cancer. However, the low density of CTCs in the peripheral blood hampers their capture and detection with high sensitivity and selectivity using currently available methods. Hence, we designed a sandwich-type electrochemical aptasensor that utilizes holothurian-shaped AuPd nanoparticles (AuPd HSs), tetrahedral DNA nanostructures (TDNs), and CuPdPt nanowire networks (NWs) interwoven with a graphdiyne (GDY) sheet for ultrasensitive non-destructive detection of MCF-7 breast cancer cells. CuPdPt NW–GDY effectively enhanced the electron transfer rate and coupled with the loaded TDNs. The TDNs could capture MCF-7 cells with precision and firmness, and the resulting composite complex was combined with AuPd HSs to form a sandwich-type structure. This novel aptasensor showed a linear range between 10 and 106 cells mL−1 and an ultralow detection limit of 7 cells mL−1. The specificity, stability, and repeatability of the measurements were successfully verified. Moreover, we used benzonase nuclease to achieve non-destructive recovery of cells for further clinical studies. According to the results, our aptasensor was more sensitive measuring the number of CTCs than other approaches because of the employment of TDNs, CuPdPt NW–GDY, and AuPd HSs. We designed a reliable sensor system for the detection of CTCs in the peripheral blood, which could serve as a new approach for cancer diagnosis at an early stage.
Graphical Abstract







Similar content being viewed by others
Data availability
The data used to support the findings of this study are included within the article.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Yamamoto A, Doak AE, Cheung KJ (2023) Orchestration of collective migration and metastasis by tumor cell clusters. Annu Rev Pathol 18:231–256. https://doi.org/10.1146/annurev-pathmechdis-031521-023557
Feng Z, Wu J, Lu Y, Chan YT, Zhang C, Wang D, Luo D, Huang Y, Feng Y, Wang N (2022) Circulating tumor cells in the early detection of human cancers. Int J Biol Sci 18(8):3251–3265. https://doi.org/10.7150/ijbs.71768
Muchlinska A, Smentoch J, Zaczek AJ, Bednarz-Knoll N (2022) Detection and characterization of circulating tumor cells using imaging flow cytometry-a perspective study. Cancers 14(17):4178. https://doi.org/10.3390/cancers14174178
Vanharanta S, Massagué J (2013) Origins of metastatic traits. Cancer Cell 24(4):410–421. https://doi.org/10.1016/j.ccr.2013.09.007
Asante DB, Calapre L, Ziman M, Meniawy TM, Gray ES (2020) Liquid biopsy in ovarian cancer using circulating tumor DNA and cells: ready for prime time. Cancer Lett 468:59–71. https://doi.org/10.1016/j.canlet.2019.10.014
Yoo TK (2021) Liquid biopsy in breast cancer: circulating tumor cells and circulating tumor DNA. Adv Exp Med Biol 1187:337–361. https://doi.org/10.1007/978-981-32-9620-6-17
Cathcart N, Chen J (2020) Sensing biomarkers with plasmonics. Anal Chem 92(11):7373–7381. https://doi.org/10.1021/acs.analchem.0c00711
He S, Yu S, Wei J, Ding L, Yang X, Wu Y (2022) New horizons in the identification of circulating tumor cells (CTCs): an emerging paradigm shift in cytosensors. Biosens Bioelectron 203:114043. https://doi.org/10.1016/j.bios.2022.114043
Zhang R, Le B, Xu W, Guo K, Sun X, Su H, Huang L, Huang J, Shen T, Liao T, Liang Y, Zhang JXJ, Dai H, Qian K (2019) Magnetic “squashing” of circulating tumor cells on plasmonic substrates for ultrasensitive NIR fluorescence detection. Small Methods 3(2):1800474. https://doi.org/10.1002/smtd.201800474
Zhao L, Liu Y, Xie SZ, Ran P, Wei JJ, Liu QJ, Li XH (2020) Janus micromotors for motion-capture-ratiometric fluorescence detection of circulating tumor cells. Chem Eng J 382:12304. https://doi.org/10.1016/j.cej.2019.123041
Cesewski E, Johnson BN (2020) Electrochemical biosensors for pathogen detection. Biosens Bioelectron 159:112214. https://doi.org/10.1016/j.bios.2020.112214
Gajdosova V, Lorencova L, Kasak P, Tkac J (2020) Electrochemical nanobiosensors for detection of breast cancer biomarkers. Sensors (Basel) 20(14):4022. https://doi.org/10.3390/s20144022
Reddy KK, Bandal H, Satyanarayana M, Goud KY, Gobi KV, Jayaramudu T, Amalraj J, Kim H (2020) Recent trends in electrochemical sensors for vital biomedical markers using hybrid nanostructured materials. Adv Sci (Weinh) 7(13):1902980. https://doi.org/10.1002/advs.201902980
Huang J, Chen X, Fu X, Li Z, Huang Y, Liang C (2021) Advances in aptamer-based biomarker discovery. Front Cell Dev Biol 9:659760. https://doi.org/10.3389/fcell.2021.659760
Alzaidy AH, Alzaidy A, Abdulsattar IN (2020) The value of two onset determination of anti-H pylori IgM antibody in patients with dyspepsia in Iraq. Int J Pharm Res 12:2. https://doi.org/10.31838/ijpr/2020.12.02.0176. (09752366)
Allah MAAH, Alshamsi HA (2023) Facile green synthesis of ZnO/AC nanocomposites using Pontederia crassipes leaf extract and their photocatalytic properties based on visible light activation. J Mater Sci-Mater Electron 34(16):1263. https://doi.org/10.1007/s10854-023-10636-y
Al-Nayili A, Idan AH (2023) Environmentally friendly production, characterization, and utilization of ZrO2 nanoparticles for the adsorption of amoxicillin in water solutions. J Mol Liq 389:122875. https://doi.org/10.1016/j.molliq.2023.122875
Yan Q, Cao L, Dong H, Tan Z, Hu Y, Liu Q, Liu H, Zhao P, Chen L, Liu Y, Li Y, Dong Y (2019) Label-free immunosensors based on a novel multi-amplification signal strategy of TiO2-NGO/Au@Pd hetero-nanostructures. Biosens Bioelectron 127:174–180. https://doi.org/10.1016/j.bios.2018.12.038
Hu W, Chang Y, Huang J, Chai Y, Yuan R (2022) Tetrahedral DNA nanostructure with multiple target-recognition domains for ultrasensitive electrochemical detection of mucin 1. Anal Chem 94(18):6860–6865. https://doi.org/10.1021/acs.analchem.2c00864
Yousefi M, Dehghani S, Nosrati R, Zare H, Evazalipour M, Mosafer J, Tehrani BS, Pasdar A, Mokhtarzadeh A, Ramezani M (2019) Aptasensors as a new sensing technology developed for the detection of MUC1 mucin: a review. Biosens Bioelectron 130:1–19. https://doi.org/10.1016/j.bios.2019.01.015
Wang DX, Wang J, Wang YX, Du YC, Huang Y, Tang AN, Cui YX, Kong DM (2021) DNA nanostructure-based nucleic acid probes: construction and biological applications. Chem Sci 12(22):7602–7622. https://doi.org/10.1039/d1sc00587a
Wan Y, Wang H, Ji J, Kang K, Yang M, Huang Y, Su Y, Ma K, Zhu L, Deng S (2020) Zippering DNA tetrahedral hyperlink for ultrasensitive electrochemical microRNA detection. Anal Chem 92(22):15137–15144. https://doi.org/10.1021/acs.analchem.0c03553
Chai H, Tang Y, Miao P (2022) Tetrahedral DNA supported walking nanomachine for ultrasensitive miRNA detection in cancer cells and serums. Anal Chem 94(28):9975–9980. https://doi.org/10.1021/acs.analchem.2c02288
Yang L, Yin X, Gai P, Li F (2020) A label-free homogeneous electrochemical cytosensor for the ultrasensitive detection of cancer cells based on multiaptamer-functionalized DNA tetrahedral nanostructures. Chem Commun (Camb) 56(27):3883–3886. https://doi.org/10.1039/d0cc00788a
Ou D, Yan H, Chen Z (2024) An impedance labeling free electrochemical aptamer sensor based on tetrahedral DNA nanostructures for doxorubicin determination. Mikrochim Acta 191(2):94. https://doi.org/10.1007/s00604-024-06176-9
Zhang XL, Liu YH, Du SM, Yin Y, Kong LQ, Chang YY, Chai YQ, Li ZH, Yuan R (2021) Engineering a rolling-circle strand displacement amplification mediated label-free ultrasensitive electrochemical biosensing platform. Anal Chem 93(27):9568–9574. https://doi.org/10.1021/acs.analchem.1c01677
Chao J, Zhu D, Zhang Y, Wang L, Fan C (2016) DNA nanotechnology-enabled biosensors. Biosens Bioelectron 76:68–79. https://doi.org/10.1016/j.bios.2015.07.007
Schlapak R, Danzberger J, Armitage D, Morgan D, Ebner A, Hinterdorfer P, Pollheimer P, Gruber HJ, Schaffler F, Howorka S (2012) Nanoscale DNA tetrahedra improve biomolecular recognition on patterned surfaces. Small 8(1):89–97. https://doi.org/10.1002/smll.201101576
Bakirhan NK, Topal BD, Ozcelikay G, Karadurmus L, Ozkan SA (2022) Current advances in electrochemical biosensors and nanobiosensors. Crit Rev Anal Chem 52(3):519–534. https://doi.org/10.1080/10408347.2020.1809339
Sharifi M, Avadi MR, Attar F, Dashtestani F, Ghorchian H, Rezayat SM, Saboury AA, Falahati M (2019) Cancer diagnosis using nanomaterials based electrochemical nanobiosensors. Biosens Bioelectron 126:773–784. https://doi.org/10.1016/j.bios.2018.11.026
Zhu Q, Hu Y, Chen H, Meng C, Shang Y, Hao C, Wei S, Wang Z, Lu X, Liu S (2023) Graphdiyne supported Ag-Cu tandem catalytic scheme for electrocatalytic reduction of CO2 to C2+ products. Nanoscale 15(5):2106–2113. https://doi.org/10.1039/d2nr05399c
Li F, Hu S, Zhang R, Gu Y, Li Y, Jia Y (2019) Porous graphene oxide enhanced aptamer specific circulating-tumor-cell sensing interface on light addressable potentiometric sensor: clinical application and simulation. ACS Appl Mater Interfaces 11(9):8704–8709. https://doi.org/10.1021/acsami.8b21101
Yuan Z, Chen J, Wen Y, Zhang C, Zhou Y, Yang Z, Yu C (2019) A trimetallic CuAuPd nanowire as a multifunctional nanocomposites applied to ultrasensitive electrochemical detection of Sema3E. Biosens Bioelectron 145:111677. https://doi.org/10.1016/j.bios.2019.111677
Wang M, Wang M, Zhan C, Geng H, Li Y, Huang X, Bu L (2022) Ultrafine platinum-iridium distorted nanowires as robust catalysts toward bifunctional hydrogen catalysis. J Mater Chem A 10(36):18972–18977. https://doi.org/10.1039/d2ta04882e
Liu R, Liu J, Jiang G (2010) Use of Triton X-114 as a weak capping agent for one-pot aqueous phase synthesis of ultrathin noble metal nanowires and a primary study of their electrocatalytic activity. Chem Commun 46(37):7010. https://doi.org/10.1039/c0cc02466j
Yin AX, Min XQ, Zhu W, Liu WC, Zhang YW, Yan CH (2012) Pt-Cu and Pt-Pd-Cu concave nanocubes with high-index facets and superior electrocatalytic activity. Chemistry 18(3):777–782. https://doi.org/10.1002/chem.201102632
Wen Y, Yuan J, Chen J, Zhao Y, Niu Y, Yu C (2017) Amperometric myeloperoxidase immunoassay based on the use of CuPdPt nanowire networks. Microchim Acta 185(1):55. https://doi.org/10.1007/s00604-017-2563-y
Kim G, Cho H, Nandhakumar P, Park JK, Kim KS, Yang H (2022) Wash-free, sandwich-type protein detection using direct electron transfer and catalytic signal amplification of multiple redox labels. Anal Chem 94(4):2163–2171. https://doi.org/10.1021/acs.analchem.1c04615
Yazdanparast S, Benvidi A, Banaei M, Nikukar H, Tezerjani MD, Azimzadeh M (2018) Dual-aptamer based electrochemical sandwich biosensor for MCF-7 human breast cancer cells using silver nanoparticle labels and a poly (glutamic acid)/MWNT nanocomposite. Microchim Acta 185(9):405. https://doi.org/10.1007/s00604-018-2918-z
Vasudevan M, Perumal V, Karuppanan S, Ovinis M, Bothi RP, Gopinath S, Immanuel ET (2022) A comprehensive review on biopolymer mediated nanomaterial composites and their applications in electrochemical sensors. Crit Rev Anal Chem 26:1–24. https://doi.org/10.1080/10408347.2022.2135090
Elahi N, Kamali M, Baghersad MH (2018) Recent biomedical applications of gold nanoparticles: a review. Talanta 184:537–556. https://doi.org/10.1016/j.talanta.2018.02.088
Huang X, Akdim O, Douthwaite M, Wang K, Zhao L, Lewis RJ, Pattisson S, Daniel IT, Miedziak PJ, Shaw G, Morgan DJ, Althahban SM, Davies TE, He Q, Wang F, Fu J, Bethell D, Mcintosh S, Kiely CJ, Hutchings GJ (2022) Au-Pd separation enhances bimetallic catalysis of alcohol oxidation. Nature 603(7900):271–275. https://doi.org/10.1038/s41586-022-04397-7
Hong W, Wang J, Wang E (2016) Scalable synthesis of Cu-based ultrathin nanowire networks and their electrocatalytic properties. Nanoscale 8(9):4927–4932. https://doi.org/10.1039/c5nr07516e
Guo J, Zhang Y, Shi L, Zhu Y, Mideksa MF, Hou K, Zhao W, Wang D, Zhao M, Zhang X, Lv J, Zhang J, Wang X, Tang Z (2017) Boosting hot electrons in hetero-superstructures for plasmon-enhanced catalysis. J Am Chem Soc 139(49):17964–17972. https://doi.org/10.1021/jacs.7b08903
He B, Lu X (2020) An electrochemical aptasensor based on tetrahedral DNA nanostructures as a signal probe carrier platform for sensitive detection of patulin. Anal Chim Acta 1138:123–131
Funding
We appreciate the support of the National Natural Science Foundation of China (No. 82102516), the Foundation of Sichuan Science and Technology Agency (No. 2023NSFSC0537), and the Graduate Innovation Fund of Chengdu Medical College (YCX2023-01–42).
Author information
Authors and Affiliations
Contributions
Hong Guo: original draft, review. Yang Fu: methodology, software. Siyu Chen: investigation, conceptualization. Linxin He: data curation, methodology. Linzhi Xie: resources, formal analysis. Mei Chen: funding acquisition, editing, and validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, H., Fu, Y., Chen, S. et al. Electrochemical cytosensor utilizing tetrahedral DNA/bimetallic AuPd holothurian-shaped nanoparticles for ultrasensitive non-destructive detection of circulating tumor cells. Microchim Acta 191, 298 (2024). https://doi.org/10.1007/s00604-024-06378-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06378-1