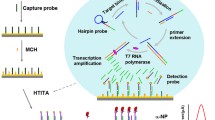
Abstract
By merging DNA entropy-driven technology with triple-stranded nucleic acids in an electrochemical biosensor to detect the SARS-CoV-2 RdRp gene, we tackled the challenges of false negatives and the high cost of SARS-CoV-2 detection. The approach generates a CRISPR-Cas 13a-activated RNA activator, which then stimulates CRISPR-Cas 13a activity using an entropy-driven mechanism. The activated CRISPR-Cas 13a can cleave Hoogsteen DNA due to the insertion of two uracil (-U-U-) in Hoogsteen DNA. The DNA tetrahedra changed on the electrode surface and can therefore not construct a three-stranded structure after cleaving Hoogsteen DNA. Significantly, this DNA tetrahedron/Hoogsteen DNA-based biosensor can regenerate at pH = 10.0, which keeps Hoogsteen DNA away from the electrode surface, allowing the biosensor to function at pH = 7.0. We could use this technique to detect the SARS-CoV-2 RdRp gene with a detection limit of 89.86 aM. Furthermore, the detection method is very stable and repeatable. This technique offers the prospect of detecting SARS-CoV-2 at a reasonable cost. This work has potential applications in the dynamic assessment of the diagnostic and therapeutic efficacy of SARS-CoV-2 infection and in the screening of environmental samples.
Graphical Abstract






Similar content being viewed by others
References
Zhu JJ (2021) Special topic: biomedical application of DNA-assembled nanostructure. J Anal Test 5(2):93–94
Pan J, He Y, Liu Z, Chen J (2022) Tetrahedron-based constitutional dynamic network for COVID-19 or other coronaviruses diagnostics and its logic gate applications. Anal Chem 94(2):714–722
Zhang K, Fan Z, Ding Y, Xie M (2022) A pH-engineering regenerative DNA tetrahedron ECL biosensor for the assay of SARS-CoV-2 RdRp gene based on CRISPR/Cas12a trans-activity. Chem Eng J 429:132472
Yu L, Zhu L, Yan M, Feng S, Huang J, Yang X (2021) Electrochemiluminescence biosensor based on entropy-driven amplification and a tetrahedral DNA nanostructure for miRNA-133a detection. Anal Chem 93(34):11809–11815
Fan Z, Yao B, Ding Y, Zhao J, Xie M, Zhang K (2021) Entropy-driven amplified electrochemiluminescence biosensor for RdRp gene of SARS-CoV-2 detection with self-assembled DNA tetrahedron scaffolds. Biosens Bioelectron 178:113015
Fan Z, Yao B, Ding Y, Xu D, Zhao J, Zhang K (2022) Rational engineering the DNA tetrahedrons of dual wavelength ratiometric electrochemiluminescence biosensor for high efficient detection of SARS-CoV-2 RdRp gene by using entropy-driven and bipedal DNA walker amplification strategy. Chem Eng J 427:131686
Zhang Y, Deng Y, Wang C, Li L, Xu L, Yu Y et al (2019) Probing and regulating the activity of cellular enzymes by using DNA tetrahedron nanostructures. Chem Sci 10(23):5959–5966
Green CM, Hastman DA, Mathur D, Susumu K, Oh E, Medintz IL et al (2021) Direct and efficient conjugation of quantum dots to DNA nanostructures with peptide-PNA. ACS Nano 15(5):9101–9110
Wang D-X, Wang J, Wang Y-X, Du Y-C, Huang Y, Tang A-N et al (2021) DNA nanostructure-based nucleic acid probes: construction and biological applications. Chem Sci 12(22):7602–7622
Wang J, Wang D-X, Ma J-Y, Wang Y-X, Kong D-M (2019) Three-dimensional DNA nanostructures to improve the hyperbranched hybridization chain reaction. Chem Sci 10(42):9758–9767
Lu J, Wang J, Hu X, Gyimah E, Yakubu S, Wang K et al (2019) Electrochemical biosensor based on tetrahedral DNA nanostructures and G-quadruplex–hemin conformation for the ultrasensitive detection of microRNA-21 in serum. Anal Chem 91(11):7353–7359
Li Z, Zhao B, Wang D, Wen Y, Liu G, Dong H et al (2014) DNA nanostructure-based universal microarray platform for high-efficiency multiplex bioanalysis in biofluids. ACS Appl Mater Inter 6(20):17944–17953
Zhao L, Qi X, Yan X, Huang Y, Liang X, Zhang L et al (2019) Engineering aptamer with enhanced affinity by triple helix-based terminal fixation. J Am Chem Soc 141(44):17493–17497
Iacovelli F, Idili A, Benincasa A, Mariottini D, Ottaviani A, Falconi M et al (2017) Simulative and experimental characterization of a pH-dependent clamp-like DNA triple-helix nanoswitch. J Am Chem Soc 139(15):5321–5329
Hu Y, Cecconello A, Idili A, Ricci F, Willner I (2017) Triplex DNA nanostructures: from basic properties to applications. Angew Chem Int Ed 56(48):15210–15233
Zhang Z, Wang Y, Zhang N, Zhang S (2016) Self-assembly of nucleic acid molecular aggregates catalyzed by a triple-helix probe for miRNA detection and single cell imaging. Chem Sci 7(7):4184–4189
Yamagata Y, Emura T, Hidaka K, Sugiyama H, Endo M (2016) Triple helix formation in a topologically controlled DNA nanosystem. Chem Eur J 22(16):5494–8
Hu F, Liu Y, Zhao S, Zhang Z, Li X, Peng N et al (2022) A one-pot CRISPR/Cas13a-based contamination-free biosensor for low-cost and rapid nucleic acid diagnostics. Biosens Bioelectron 202:113994
Zhou T, Huang M, Lin J, Huang R, Xing D (2021) High-fidelity CRISPR/Cas13a trans-cleavage-triggered rolling circle amplified DNAzyme for visual profiling of microRNA. Anal Chem 93(4):2038–2044
Liu X, Kang X, Lei C, Ren W, Liu C (2022) Programming the trans-cleavage activity of CRISPR-Cas13a by single-strand DNA blocker and its biosensing application. Anal Chem 94(9):3987–3996
Zhang K, Fan Z, Ding Y, Zhu S, Xie M, Hao N (2022) Exploring the entropy-driven amplification reaction and trans-cleavage activity of CRISPR-Cas12a for the development of an electrochemiluminescence biosensor for the detection of the SARS-CoV-2 RdRp gene in real samples and environmental surveillance. Environ Sci Nano 9(1):162–172
Lee I, Kwon S-J, Sorci M, Heeger PS, Dordick JS (2021) Highly sensitive immuno-CRISPR assay for CXCL9 detection. Anal Chem 93(49):16528–16534
Chen Q, Tian T, Xiong E, Wang P, Zhou X (2020) CRISPR/Cas13a signal amplification linked immunosorbent assay for femtomolar protein detection. Anal Chem 92(1):573–577
Ramachandran A, Santiago JG (2021) CRISPR enzyme kinetics for molecular diagnostics. Anal Chem 93(20):7456–7464
Zhao J, Tan Z, Wang L, Lei C, Nie Z (2021) A ligation-driven CRISPR–Cas biosensing platform for non-nucleic acid target detections. Chem Commun 57(57):7051–7054
Liang M, Li Z, Wang W, Liu J, Liu L, Zhu G, et al (2019) A CRISPR-Cas12a-derived biosensing platform for the highly sensitive detection of diverse small molecules. Nat Commun 10
Suea-Ngam A, Howes PD, deMello AJ (2021) An amplification-free ultra-sensitive electrochemical CRISPR/Cas biosensor for drug-resistant bacteria detection. Chem Sci 12(38):12733–12743
Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J et al (2017) Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 356(6336):438–442
Zhang K, Zhou L, Zhang T, Fan Z, Xie M, Ding Y et al (2021) Peptide based biosensing of protein functional control indicates novel mechanism of cancerous development under oxidative stress. Sens Actuators B Chem 329:129121
Zhang K, Li J, Fan Z, Li H, Xu J-J (2021) “Covalent biosensing” enables a one-step, reagent-less, low-cost and highly robust assay of SARS-CoV-2. Chem Commun 57(82):10771–10774
Xu D, Xu X, Fan Z, Zou M, Qin X, Ding Y et al (2021) Hypersensitive detection of transcription factors by multiple amplification strategy based on molecular beacon. Microchem J 171:106837
Zhang K, Fan Z, Huang Y, Ding Y, Xie M (2022) A strategy combining 3D-DNA Walker and CRISPR-Cas12a trans-cleavage activity applied to MXene based electrochemiluminescent sensor for SARS-CoV-2 RdRp gene detection. Talanta 236:122868
Tao X-L, Pan M-C, Yang X, Yuan R, Zhuo Y (2022) CDs assembled metal-organic framework: exogenous coreactant-free biosensing platform with pore confinement-enhanced electrochemiluminescence. Chin Chem Lett 33(11):4803–4807
Zhao H, Su E, Huang L, Zai Y, Liu Y, Chen Z et al (2022) Washing-free chemiluminescence immunoassay for rapid detection of cardiac troponin I in whole blood samples. Chin Chem Lett 33(2):743–746
Zhang K, Fan Z, Ding Y, Li J, Li H (2021) Thiol-sensitive probe enables dynamic electrochemical assembly of serum protein for detecting SARS-Cov-2 marker protease in clinical samples. Biosens Bioelectron 194:113579
Fan Z, Ding Y, Yao B, Wang J, Zhang K (2021) Electrochemiluminescence platform for transcription factor diagnosis by using CRISPR–Cas12a trans-cleavage activity. Chem Commun 57(65):8015–8018
Kumar N, Shetti NP, Jagannath S, Aminabhavi TM (2022) Electrochemical sensors for the detection of SARS-CoV-2 virus. Chem Eng J 430:132966
Fan Z, Xie M, Pan J, Zhang K (2022) SARS-CoV-2 monitoring by automated target-driven molecular machine based engineering. Environ Chem Lett 20 (4):2227–2233
Das D, Lin C-W, Kwon JS, Chuang HS (2022) Rotational diffusometric sensor with isothermal amplification for ultra-sensitive and rapid detection of SARS-CoV-2 nsp2 cDNA. Biosens Bioelectron 210:114293
Zhao H, Liu F, Xie W, Zhou T, OuYang J, Jin L et al (2021) Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens Actuators B Chem 327:128899
Zhou T, Huang R, Huang M, Shen J, Shan Y, Xing D (2020) CRISPR/Cas13a powered portable electrochemiluminescence chip for ultrasensitive and specific MiRNA detection. Adv Sci 7(13):1903661
Funding
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (21964018, 81860851), Guangxi Medical High-level Leading Talents Training 139 Project (GWKJ2018-22), Special Funding for Guangxi Special Experts (GRCT2019-13), the Natural Science Foundation of Guangxi Province (2018GXNSFDA281017), the Jiangsu Provincial Health Care Commission Scientific Research Project (M2021035), and the Wuxi “Taihu Light” science and technology (medical and health technology) research (Y20212049).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhuo, C., Song, Z., Cui, J. et al. Electrochemical biosensor strategy combining DNA entropy-driven technology to activate CRISPR-Cas13a activity and triple-stranded nucleic acids to detect SARS-CoV-2 RdRp gene. Microchim Acta 190, 272 (2023). https://doi.org/10.1007/s00604-023-05848-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05848-2