Abstract
A nanoplatform based on metal–organic frameworks (MOFs) and lambda exonuclease (λ exo) for the fluorimetric determination of T4 polynucleotide kinase (T4 PNK) activity and inhibition is described. Fe-MIL-88 was selected as the nanomaterial because of its significant preferential binding ability to single-stranded DNA (ssDNA) over double-stranded DNA (dsDNA) and its quenching property. The synthesized Fe-MIL-88 was characterized by transmission electron microscope, scanning electron microscope, and X-ray photoelectron spectroscopy. In the presence of T4 PNK, FAM-labeled dsDNA (FAM-dsDNA) is phosphorylated on its 5′-terminal. λ exo then recognizes and cleaves the phosphorylated strand yielding FAM-labeled ssDNA (FAM-ssDNA). The fluorescence of the produced FAM-ssDNA is quenched due to Fe-MIL-88’s absorbing on FAM-ssDNA. On the contrary, in the absence of T4 PNK, the phosphorylation and cleavage processes cannot take place. Therefore, the fluorescence of FAM-dsDNA still remains. The fluorescence intensity is detected at the maximum emission wavelength of 524 nm using the maximum excitation wavelength of 488 nm. The assay of T4 PNK based on the fluorescence quenching of FAM-ssDNA achieves a linear relationship in the range 0.01–5.0 U mL−1 with a detection limit of 0.0089 U mL−1 in buffer. The assay exhibits excellent performance for T4 PNK activity determination in a complex biological matrix. The results also reveal the ability of the assay for T4 PNK inhibitor screening.

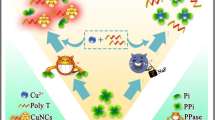
Schematic presentation of a nanoplatform based on Fe-MIL-88 and coupled exonuclease reaction for the fluorimetric determination of T4 polynucleotide kinase activity. FAM-ssDNA, FAM-labeled single-stranded DNA; cDNA, complementary DNA; λ exo, lambda exonuclease;T4 PNK, T4 polynucleotide kinase







Similar content being viewed by others
References
Whitehouse CJ, Taylor RM, Thistlethwaite A, Zhang H, Karimi-Busheri F, Lasko DD, Weinfeld M, Caldecott KW (2001) XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell 104:107–117. https://doi.org/10.1016/s0092-8674(01)00195-7
Bernstein NK, Williams RS, Rakovszky ML, Cui D, Green R, Karimi-Busheri F, Mani RS, Galicia S, Koch CA, Cass CE, Durocher D, Weinfeld M, Glover JNM (2005) The molecular architecture of the mammalian DNA repair enzyme, polynucleotide kinase. Mol Cell 17:657–670. https://doi.org/10.1016/j.molcel.2005.02.012
Lin L, Liu Y, Yan J, Wang X, Li J (2013) Sensitive nanochannel biosensor for T4 polynucleotide kinase activity and inhibition detection. Anal Chem 85:334–340. https://doi.org/10.1021/ac302875p
Jiang HX, Kong DM, Shen HX (2014) Amplified detection of DNA ligase and polynucleotide kinase/phosphatase on the basis of enrichment of catalytic G-quadruplex DNAzyme by rolling circle amplification. Biosens Bioelectron 55:133–138. https://doi.org/10.1016/j.bios.2013.12.001
Song W, Yin W, Zhang Z, He P, Yang X, Zhang X (2019) A DNA functionalized porphyrinic metal-organic framework as a peroxidase mimicking catalyst for amperometric determination of the activity of T4 polynucleotide kinase. Microchim Acta 186(3):149. https://doi.org/10.1007/s00604-019-3269-0
Xu XM, Cen Y, Xu GH, Wei FD, Shi ML, Hu Q (2019) A ratiometric fluorescence probe based on carbon dots for discriminative and highly sensitive detection of acetylcholinesterase and butyrylcholinesterase in human whole blood. Biosens Bioelectron 131:232–236. https://doi.org/10.1016/j.bios.2019.02.031
Cen Y, Wu YM, Kong XJ, Wu S, Yu RQ, Chu X (2014) Phospholipid-modified upconversion nanoprobe for ratiometric fluorescence detection and imaging of phospholipase D in cell lysate and in living cells. Anal Chem 86:7119–7127. https://doi.org/10.1021/ac5016694
Li J, Ma J, Zhang Y, Zhang Z, He G (2019) A fluorometric method for determination of the activity of T4 polynucleotide kinase by using a DNA-templated silver nanocluster probe. Microchim Acta 186(1):48. https://doi.org/10.1007/s00604-018-3157-z
Zhang XX, Liu Q, Jin Y, Li BX (2019) Determination of the activity of T4 polynucleotide kinase phosphatase by exploiting the sequence-dependent fluorescence of DNA-templated copper nanoclusters. Microchim Acta 186(1):3–9. https://doi.org/10.1007/s00604-018-3102-1
Cook TR, Zheng Y-R, Stang PJ (2013) Metal-organic frameworks and self-assembled supramolecular coordination complexes: comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem Rev 113:734–777. https://doi.org/10.1021/cr3002824
Wang B, Cote AP, Furukawa H, O'Keeffe M, Yaghi OM (2008) Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs. Nature 453:207–U206. https://doi.org/10.1038/nature06900
Mon M, Tiburcio E, Ferrando-Soria, Gil San Millan R, Navarro JAR, Armentano D, Pardo E (2018) A post-synthetic approach triggers selective and reversible sulphur dioxide adsorption on a metal-organic framework. Chem Commun 54:9063–9066. https://doi.org/10.1039/c8cc04482a
Ma X, Wen S, Xue X, Guo Y, Jin J, Song W, Zhao B (2018) Controllable synthesis of SERS-active magnetic metal-organic framework-based nanocatalysts and their application in photoinduced enhanced catalytic oxidation. ACS Appl Mater Interfaces 10:25726–25736. https://doi.org/10.1021/acsami.8b03457
Chen WH, Luo GF, Vazquez-Gonzalez M, Cazelles R, Sohn YS, Nechushtai R, Mandel Y, Willner I (2018) Glucose-responsive metal-organic-framework nanoparticles act as “smart” sense-and-treat carriers. ACS Nano 12:7538–7545. https://doi.org/10.1021/acsnano.8b03417
Wang Y, He X, Wang K, Ni X, Su J, Chen Z (2011) Ferrocene-functionalized SWCNT for electrochemical detection of T4 polynucleotide kinase activity. Biosens Bioelectron 32:213–218. https://doi.org/10.1016/j.bios.2011.12.012
Peng Y, Jiang J, Yu R (2013) An electrochemical assay of polynucleotide kinase activity based on streptavidin–gold nanoparticles and enzymatic amplification. RSC Adv 3:18128–18133. https://doi.org/10.1039/c3ra43315c
Zhu C, Zeng Z, Li H, Li F, Fan C, Zhang H (2013) Single-layer MoS2-based nanoprobes for homogeneous detection of biomolecules. J Am Chem Soc 135:5998–6001. https://doi.org/10.1021/ja4019572
Lin L, Liu Y, Zhao X, Li J (2011) Sensitive and rapid screening of T4 polynucleotide kinase activity and inhibition based on coupled exonuclease reaction and graphene oxide platform. Anal Chem 83:8396–8402. https://doi.org/10.1021/ac200593g
Zhang HT, Zhang JW, Huang G, Du ZY, Jiang HL (2014) An amine-functionalized metal-organic framework as a sensing platform for DNA detection. Chem Commun 50:12069–12072. https://doi.org/10.1039/c4cc05571c
Xie S, Ye J, Yuan Y, Chai Y, Yuan R (2015) A multifunctional hemin@metal-organic framework and its application to construct an electrochemical aptasensor for thrombin detection. Nanoscale 7:18232–18238. https://doi.org/10.1039/c5nr04532k
Li X, Guo W, Liu Z, Wang R, Liu H (2016) Fe-based MOFs for efficient adsorption and degradation of acid orange 7 in aqueous solution via persulfate activation. Appl Surf Sci 369:130–136. https://doi.org/10.1016/j.apsusc.2016.02.037
Smith BC (2016) Distinguishing structural isomers: mono- and disubstituted benzene rings. Spectroscopy 31:36–39
Zu F, Yan F, Bai Z, Xu J, Wang Y, Huang Y, Zhou X (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184:1899–1914. https://doi.org/10.1007/s00604-017-2318-9
Wang L, Zhang Q, Tang B, Zhang C (2017) Single-molecule detection of polynucleotide kinase based on phosphorylation-directed recovery of fluorescence quenched by au nanoparticles. Anal Chem 89:7255–7261. https://doi.org/10.1021/acs.analchem.7b01783
Zhao H, Yan Y, Chen M, Hu T, Wu K, Liu H, Ma C (2019) Exonuclease III-assisted signal amplification strategy for sensitive fluorescence detection of polynucleotide kinase based on poly(thymine)-templated copper nanoparticles. Analyst 144:6689–6697. https://doi.org/10.1039/c9an01659g
Zhuang J, Lai W, Xu M, Zhou Q, Tang D (2015) Plasmonic AuNP/g-C3N4 nanohybrid-based photoelectrochemical sensing platform for ultrasensitive monitoring of polynucleotide kinase activity accompanying DNAzyme-catalyzed precipitation amplification. ACS Appl Mater Interfaces 7:8330–8338. https://doi.org/10.1021/acsami.5b01923
Gao M, Guo J, Song Y, Zhu Z, Yang CJ (2017) Detection of T4 polynucleotide kinase via allosteric aptamer probe platform. ACS Appl Mater Interfaces 9:38356–38363. https://doi.org/10.1021/acsami.7b14185
Guo Y, Wang Q, Wang Z, Chen X, Xu L, Hu J, Pei R (2015) Label-free detection of T4 DNA ligase and polynucleotide kinase activity based on toehold-mediated strand displacement and split G-quadruplex probes. Sensor Actuat B-Chem 214:50–55. https://doi.org/10.1016/j.snb.2015.03.013
Cen Y, Deng WJ, Yang Y, Yu RQ, Chu X (2017) Core-shell-shell multifunctional nanoplatform for intracellular tumor-related mRNAs imaging and near-infrared light triggered photodynamic-photothermal synergistic therapy. Anal Chem 89:10321–10328. https://doi.org/10.1021/acs.analchem.7b02081
Allinson SL (2010) DNA end-processing enzyme polynucleotide kinase as a potential target in the treatment of cancer. Future Oncol 6:1031–1042. https://doi.org/10.2217/fon.10.40
Feng C, Wang Z, Chen T, Chen X, Mao D, Zhao J, Li G (2018) A dual-enzyme-assisted three-dimensional DNA walking machine using T4 polynucleotide kinase as activators and application in polynucleotide kinase assays. Anal Chem 90:2810–2815. https://doi.org/10.1021/acs.analchem.7b04924
Lillehaug JR, Kleppe K (1975) Effect of salts and polyamines on T4 polynucleotide kinase. Biochem 14:1225–1229. https://doi.org/10.1021/bi00677a021
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 61775099, 81973283, 21705080) and Natural Science Foundation of Jiangsu Province (No. BK20171487, BK20171043), Science and Technology Development Fund of Nanjing Medical University-Major Project (No. NMUD2018004) and R&D fund for Smart Health Technology Innovation of Nanjing Medical University and Jiangsu Salt Group (No. NMU-SY201801).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 5386 kb)
Rights and permissions
About this article
Cite this article
Chai, Y., Cheng, X., Xu, G. et al. A nanoplatform based on metal–organic frameworks and coupled exonuclease reaction for the fluorimetric determination of T4 polynucleotide kinase activity and inhibition. Microchim Acta 187, 243 (2020). https://doi.org/10.1007/s00604-020-4194-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4194-y