Abstract
The study presents the synthesis of polypyrrole-coated palladium platinum/nitrogen-doped reduced graphene oxide nanocomposites (PdPt-PPy/N-rGO NC) via direct the reduction of Pd(II) and Pt(II) in the presence of pyrrole monomer, N-rGO and L-cysteine as the reducing agent. X-ray diffraction confirmed the presence of metallic Pd and Pt from the reduction of Pd and Pt cations. Transmission electron microscopy images revealed the presence of Pd, Pt and PPy deposition on N-rGO. Impedance spectroscopy results gave a decreased charge transfer resistance due to the presence of N-rGO. The nanocomposites were synthesized with different Pd/Pt ratios (2:1, 1:1 and 1:2). A glassy carbon electrode (GCE) modified with the nanocomposite showed enhanced electrochemical sensing capability for formaldehyde in 0.1 M sulfuric acid solution. Cyclic voltammetry showed an increase in the formaldehyde oxidation peak current at the GCE modified with Pd2Pt1 PPy N-rGO. At a typical potential of 0.45 V (vs. SCE), the sensitivity in the linear segment was 345.8 μA.mM −1. cm−2. The voltammetric response was linear between 0.01 and 0.9 mM formaldehyde concentration range, with a 27 µM detection limit (at S/N = 3).

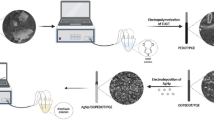
Schematic presentation of formaldehyde detection by Pd2Pt1-PPy/nitrogen-doped reduced Graphene Oxide Nanocomposite (Pd2Pt1-PPy /N-Gr NC). The decrease of charge transfer resistance and the agglomeration of deposited metals in the presence of N-rGO enhance the current response of the electrochemical sensor.







Similar content being viewed by others
References
Wang J, Pamidi PVA, Cepria G (1996) Electrocatalysis and amperometric detection of aliphatic aldehydes at platinum–palladium alloy coated glassy carbon electrode. Anal Chim Acta 330:151–158
Vianello F, Stefani A, Di Paolo ML, Rigo A, Lui A, Margesin B, Zen M, Scarpa M, Soncini G (1996) Potentiometric detection of formaldehyde in air by an aldehyde dehydrogense FET. Sensors Actuators B Chem 37:49–54
Herschkoviz Y, Eshkenazi I, Campbell CE, Rishpon J (2000) An electrochemical biosensor for formaldehyde. J Electroanal Chem 491:182–187
Mohlmann GR (1985) Formaldehyde detection in air by laser induced fluorescence. Appl Spectrosc 39:98–101
Mann B, Grajeski ML (1987) New chemiluminescent derivatizing agent for the analysis of aldehydes and ketones by high-performance liquid chromatography with peroxioxalate chemiluminescence. J Chromatogr 386:149–158
Dumas T (1982) Determination of formaldehyde in air by gas chromatography. J Chromatogr 247:289–295
Zhou ZL, Kang TF, Zhang Y, Cheng SY (2009) Electrochemical sensor for formaldehyde based on Pt–Pd nanoparticles and a Nafion-modified glassy carbon electrode. Microchim Acta 164:133–138
Ashrafi A, Golozar MA, Mallakpour S (2006) Morphological investigations of polypyrrole coatings on stainless. Synth Met 156:1280–1285
Kang X, Wang J, Wu H, Aksay IA, Liu J, Lin Y (2009) Glucose oxidase–graphene– chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens Bioelectron 25:901–905
Zang J, Li X (2011) In situ synthesis of ultrafine b-MnO2/polypyrrole nanorod composites for high-performance supercapacitors. J Mater Chem 21:10965–10969
Fan Y, Liu JH, Yang CP, Yu M, Liu P (2011) Graphene–polyaniline composite film modified electrode for voltammetric determination of 4-aminophenol. Sensors Actuators B Chem 157:669–674
Luo Y, Lu W, Chang G, Liao F, Sun X (2011) One-step preparation of ag nanoparticle-decorated coordination polymer nanobelts and their application for enzymeless H2O2 detection. Electrochim Acta 56:8371–8374
Reghu M, Cao Y, Moses D, Heeger AJ (1993) Counterion-induced processibility of polyaniline: transport at the metal-insulator boundary. Phys Rev B 47:1758–1764
Peng Y, Lin D, Justin Gooding J, Xue Y, Dai L (2018) Flexible fiber-shaped non-enzymatic sensors with a graphene-metal heterostructure based on graphene fibres decorated with gold nanosheets. Carbon 136:329–236
Khodadadi Z (2018) Evaluation of H2S sensing characteristics of metals –doped graphene and metals decorated graphene: insights from DFT study. Physica E: Low-Dimens Syst Nanostruct 99:261–268
Mahmoudian MR, Alias Y, Basirun W, Woi PM, Sookhakian M (2014) Facile preparation of MnO2 nanotubes/reduced graphene oxide nanocomposite for electrochemical sensing of hydrogen peroxide. Sensors Actuators B Chem 201:526–534
Baradaran S, Moghaddam E, Nasiri-Tabrizi B, Basirun W, Mehrali M, Sookhakian M, Hamdi M, Alias Y (2015) Characterization of nickel-doped biphasic calcium phosphate/graphene nanoplatelet composites for biomedical application. Mater Sci Eng C Mater Biol Appl 49:656–668
Sookhakian M, Amin YM, Zakaria R, Baradaran S, Mahmoudian MR, Rezayi M, Tajabadi MT, Basirun WJ (2014) Enhanced photovoltaic performance of polymer hybrid nanostructure heterojunction solar cells based on poly (3-hexylthiophene)/ZnS/ZnO/reduced graphene oxide shell–core nanorod arrays. Ind Eng Chem Res 53:14301–14309
Sookhakian M, Ridwan N, Zalnezhad E, Yoon G, Azarang M, Mahmoudian MR, Alias Y (2016) Layer-by-layer electrodeposited reduced graphene oxide- copper Nanopolyhedra films as efficient platinum-free counter electrodes in high efficiency dye-sensitized solar cells. J Electrochem Soc 163:D154–D159
Tajabadi MT, Basirun WJ, Lorestani F, Zakaria R, Baradaran S, Amin Y, Mahmoudian MR, Rezayi M, Sookhakian M (2015) Nitrogen-doped graphene-silver nanodendrites for the non-enzymatic detection of hydrogen peroxide. Electrochim Acta 151:126–133
Shan C, Yang H, Song J, Han D, Ivaska A, Niu L (2009) Direct electrochemistry ofglucose oxidase and biosensing for glucose based on graphene. Anal Chem 81:2378–2382
Lim HN, Huang NM, Lim SS, Harrison I, Chia CH (2011) Simple room-temperature preparation of high-yield large-area graphene oxide. Int J Nanomedicine 6:3443–3448
Jin J, Fu X, Liu Q, Liu Y, Wei Z, Niu K, Zhang J (2013) Identifying the active site in nitrogen-doped graphene for the VO2+/VO2+ redox reaction. ACS Nano 7:4764–4773
Yamauchi O, Odani A, Takani M (2002) Metal–amino acid chemistry. Weak interactions and related functions of side chain groups. J Chem Soc Dalton Trans 18:3411–3421
Selvaraj V, Alagar M, Kumar KS (2007) Synthesis and characterization of metal nanoparticles-decorated PPY-CNT composite and their electrocatalytic oxidation of formic acid and formaldehyde for fuel cell applications. Appl Catal B Environ 75:129–138
Randles JEB (1948) A cathode ray polarograph. Part II.—the current-voltage curves. Trans Faraday Soc 44:327–338
Wilke CR, Chang P (1955) Correlation of diffusion coefficients in dilute solutions. AICHE J 1:264–270
Lian W, Wang L, Song Y, Yuan H, Zhao S, Li P, Chen L (2009) A hydrogen peroxide sensorbased on electrochemically roughened silver electrodes. Electrochim Acta 54:4334–4339
Pinto GF, Rocha DP, Richter EM, Muñoz RAA, Silva SG (2019) Indirect determination of formaldehyde by square-wave voltammetry based on the electrochemical oxidation of 3,5–diacetyl–1,4–dihydrolutidine using an unmodified glassy-carbon electrode. Talanta 198:237–241
Trivedi D, Crosse J, Tanti J, Cass AJ, Toghill KE (2018) The electrochemical determination of formaldehyde in aqueous media using nickel modified electrodes. Sensors Actuators B Chem 270:298–303
Zhang Y, Zhang M, Cai Z, Chen M, Cheng F (2012) A novel electrochemical sensor for formaldehyde based on palladium nanowire arrays electrode in alkaline media. Electrochim Acta 68:172–177
Chi X, Liu C, Liu L, Li S, Li H, Zhang X, Bo X, Shan H (2014) Enhanced formaldehyde-sensing properties of mixed Fe2O3 –In2O3 nanotubes. Mater Sci Semicond Process 18:160–164
Zheng Y, Wang J, Yao P (2011) Formaldehyde sensing properties of electrospun NiO-doped SnO2 nanofibers. Sensors Actuators B Chem 156:723–730
Mei H, Wu W, Yu B, Wu H, Xia Q (2016) Nonenzymatic electrochemical sensor based on Fe@Pt core–shell nanoparticles for hydrogen peroxide, glucose and formaldehyde. Sensors Actuators B Chem 223:68–75
Acknowledgments
The authors wish to thank Mojdeh Yeganeh for valuable discussion. This research is supported by the UMRG Sub program RP020C-16SUS and Frontier Research Grant FG030-17AFR.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.60 mb)
Rights and permissions
About this article
Cite this article
Mahmoudian, M.R., Basirun, W.J., Woi, P.M. et al. Voltammetric sensing of formaldehyde by using a nanocomposite prepared by reductive deposition of palladium and platinum on polypyrrole-coated nitrogen-doped reduced graphene oxide. Microchim Acta 186, 369 (2019). https://doi.org/10.1007/s00604-019-3481-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3481-y