Abstract
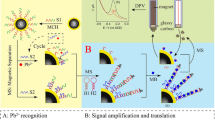
Two-dimensional (2D) MoS2 is found to possess different affinities for ssDNA and dsDNA. This finding is exploited in an amperometric aptamer-based method for the determination of the mycotoxin ochratoxin A (OTA). Initially, a dsDNA probe (formatted through the hybridization of OTA-aptamer with an auxiliary DNA) is self-assembled on a gold electrode. Upon introduction of OTA, it will bind to the aptamer and cause the unwinding of dsDNA, while the auxiliary DNA (with single-stranded structure) remains on the electrode. Since the affinity of 2D MoS2 for ssDNA is considerably larger than that for dsDNA, it will be adsorbed on the electrode by binding to the auxiliary DNA. Notably, 2D MoS2 possesses peroxidase-like activity. Hence, it can catalyze the amplification of electrochemical signal of the hydroquinone/benzoquinone redox system. Under optimal conditions, the amperometric signal (best measured at −0.2 V vs. SCE) increases with increasing OTA concentration in the range from 0.5 pg·mL−1 to 1.0 ng·mL−1, with a lower detection limit of 0.23 pg·mL−1. The method was applied to the determination of OTA in spiked red wine.

Herein we construct a convenient electrochemical aptasensor for sensitive monitor of ochratoxin A by using 2D MoS2 as a nano-binder to catalyze the amplification of electrochemical signal from hydroquinone/benzoquinone system.



Similar content being viewed by others
References
Breitholtz A, Olsen M, Dahlbäck Å, Hult K (1991) Plasma ochratoxin A levels in three Swedish populations surveyed using an ion-pair HPLC technique. Food additives and Contaminats 8:183–192
Qing Y, Li X, Chen S, Zhou X, Luo M, Xu X, Li C, Qiu J (2017) Differential pulse voltammetric ochratoxin A assay based on the use of an aptamer and hybridization chain reaction. Microchim Acta 184:863–870
Abnous K, Danesh NM, Alibolandi M, Ramezani M, Taghdisi SM (2017) Amperometric aptasensor for ochratoxin A based on the use of a gold electrode modified with aptamer, complementary DNA, SWCNTs and the redox marker Methylene Blue. Microchim Acta 184:1151–1159
Zhang C, Tang J, Huang L, Li Y, Tang D (2017) In-situ amplified voltammetric immunoassay for ochratoxin A by coupling a platinum nanocatalyst based enhancement to a redox cycling process promoted by an enzyme mimic. Microchim Acta 184:2445–2453
Jalili M, Jinap S (2012) Natural occurrence of aflatoxins and ochratoxin A in commercial dried chili. Food Control 24:160–164
Chen X, Wu G, Cai Z, Oyama M, Chen X (2014) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181:689–705
Yang C, Wang Y, Marty J-L, Yang X (2011) Aptamer-based colorimetric biosensing of Ochratoxin A using unmodified gold nanoparticles indicator. Biosens Bioelectron 26:2724–2727
Flajs D, Domijan AM, Ivić D, Cvjetković B, Peraica M (2009) ELISA and HPLC analysis of ochratoxin A in red wines of Croatia. Food Control 20:590–592
Han X, Zhang Y, Nie J, Zhao S, Tian Y, Zhou N (2017) Gold nanoparticle based photometric determination of tobramycin by using new specific DNA aptamers. Microchim Acta 185:4
Nasirian V, Chabok A, Barati A, Rafienia M, Arabi MS, Shamsipur M (2017) Ultrasensitive aflatoxin B1 assay based on FRET from aptamer labelled fluorescent polymer dots to silver nanoparticles labeled with complementary DNA. Microchim Acta 184:4655–4662
Weng X, Neethirajan S (2017) Aptamer-based fluorometric determination of norovirus using a paper-based microfluidic device. Microchim Acta 184:4545–4552
Wu J, Zhu Y, Xue F, Mei Z, Yao L, Wang X, Zheng L, Liu J, Liu G, Peng C, Chen W (2014) Recent trends in SELEX technique and its application to food safety monitoring. Microchim Acta 181:479–491
Ren X, Yan J, Wu D, Wei Q, Wan Y (2017) Nanobody-Based Apolipoprotein E Immunosensor for Point-of-Care Testing. ACS Sensors 2:1267–1271
Han Q, Wang R, Xing B, Zhang T, Khan MS, Wu D, Wei Q (2018) Label-free photoelectrochemical immunoassay for CEA detection based on CdS sensitized WO3@BiOI heterostructure nanocomposite. Biosens Bioelectron 99:493–499
Wang X, Xu R, Sun X, Wang Y, Ren X, Du B, Wu D, Wei Q (2017) Using reduced graphene oxide-Ca:CdSe nanocomposite to enhance photoelectrochemical activity of gold nanoparticles functionalized tungsten oxide for highly sensitive prostate specific antigen detection. Biosens Bioelectron 96:239–245
Taleat Z, Khoshroo A, Mazloum-Ardakani M (2014) Screen-printed electrodes for biosensing: a review (2008–2013). Microchim Acta 181:865–891
Dai S, Wu S, Duan N, Chen J, Zheng Z, Wang Z (2017) An ultrasensitive aptasensor for Ochratoxin A using hexagonal core/shell upconversion nanoparticles as luminophores. Biosens Bioelectron 91:538–544
Dai S, Wu S, Duan N, Wang Z (2016) A luminescence resonance energy transfer based aptasensor for the mycotoxin Ochratoxin A using upconversion nanoparticles and gold nanorods. Microchim Acta 183:1909–1916
Wang S, Zhang Y, Pang G, Zhang Y, Guo S (2017) Tuning the Aggregation/Disaggregation Behavior of Graphene Quantum Dots by Structure-Switching Aptamer for High-Sensitivity Fluorescent Ochratoxin A Sensor. Anal Chem 89:1704–1709
Xie S, Chai Y, Yuan Y, Bai L, Yuan R (2014) Development of an electrochemical method for Ochratoxin A detection based on aptamer and loop-mediated isothermal amplification. Biosens Bioelectron 55:324–329
Radisavljevic B, Radenovic A, Brivio J, Giacometti iV, Kis A (2011) Single-layer MoS2 transistors. Nat Nanotechnol 6:147–14s
Cheng Z, Shen Q, Yu H, Han D, Zhong F, Yang Y (2017) Non-enzymatic sensing of hydrogen peroxide using a glassy carbon electrode modified with the layered MoS2-reduced graphene oxide and Prussian Blue. Microchim Acta 184:4587–4595
Gan X, Zhao H, Quan X (2017) Two-dimensional MoS2: A promising building block for biosensors. Biosens Bioelectron 89(Part 1):56–71
Singh P, Gupta R, Sinha M, Kumar R, Bhalla V (2016) MoS2 based digital response platform for aptamer based fluorescent detection of pathogens. Microchim Acta 183:1501–1506
Qu F, Liu Y, Kong R, You J (2017) A versatile DNA detection scheme based on the quenching of fluorescent silver nanoclusters by MoS2 nanosheets: Application to aptamer-based determination of hepatitis B virus and of dopamine. Microchim Acta 184:4417–4424
Cao X (2014) Ultra-sensitive electrochemical DNA biosensor based on signal amplification using gold nanoparticles modified with molybdenum disulfide, graphene and horseradish peroxidase. Microchim Acta 181:1133–1141
Yang Y, Zhang H, Huang C, Yang D, Jia N (2017) Electrochemical non-enzyme sensor for detecting clenbuterol (CLB) based on MoS2-Au-PEI-hemin layered nanocomposites. Biosensors and Bioelectronics 89. Part 1:461–467
Shu Y, Chen J, Xu Q, Wei Z, Liu F, Lu R, Xu S, Hu X (2017) MoS2 nanosheet-Au nanorod hybrids for highly sensitive amperometric detection of H2O2 in living cells. J Mater Chem B 5:1446–1453
Wang X, Chu C, Shen L, Deng W, Yan M, Ge S, Yu J, Song X (2015) An ultrasensitive electrochemical immunosensor based on the catalytical activity of MoS2-Au composite using Ag nanospheres as labels. Sensors Actuators B 206:30–36
Wang Y, Ma T, Ma S, Liu Y, Tian Y, Wang R, Jiang Y, Hou D, Wang J (2017) Fluorometric determination of the antibiotic kanamycin by aptamer-induced FRET quenching and recovery between MoS2 nanosheets and carbon dots. Microchim Acta 184:203–210
Zhu C, Zeng Z, Li H, Li F, Fan C, Zhang H (2013) Single-Layer MoS2-Based Nanoprobes for Homogeneous Detection of Biomolecules. J Am Chem Soc 135:5998–6001
Wang X, Nan F, Zhao J, Yang T, Ge T, Jiao K (2015) A label-free ultrasensitive electrochemical DNA sensor based on thin-layer MoS2 nanosheets with high electrochemical activity. Biosens Bioelectron 64:386–391
Jia L, Ding L, Tian J, Bao L, Hu Y, Ju H, Yu J-S (2015) Aptamer loaded MoS2 nanoplates as nanoprobes for detection of intracellular ATP and controllable photodynamic therapy. Nano 7:15953–15961
Kenry GA, Zhang X, Zhang H, Lim CT (2016) Highly Sensitive and Selective Aptamer-Based Fluorescence Detection of a Malarial Biomarker Using Single-Layer MoS2 Nanosheets. ACS Sensors 1:1315–1321
Lin T, Zhong L, Guo L, Fu F, Chen G (2014) Seeing diabetes: visual detection of glucose based on the intrinsic peroxidase-like activity of MoS2 nanosheets. Nano 6:11856–11862
Lu Y, Yu J, Ye W, Yao X, Zhou P, Zhang H, Zhao S, Jia L (2016) Spectrophotometric determination of mercury(II) ions based on their stimulation effect on the peroxidase-like activity of molybdenum disulfide nanosheets. Microchim Acta 183:2481–2489
Zhu Z, Feng M, Zuo L, Zhu Z, Wang F, Chen L, Li J, Shan G, Luo S-Z (2015) An aptamer based surface plasmon resonance biosensor for the detection of ochratoxin A in wine and peanut oil. Biosens Bioelectron 65:320–326
Zhang J, Zhang X, Yang G, Chen J, Wang S (2013) A signal-on fluorescent aptasensor based on Tb3+ and structure-switching aptamer for label-free detection of Ochratoxin A in wheat. Biosens Bioelectron 41:704–709
L-h L, X-h Z, H-c S (2015) Portable optical aptasensor for rapid detection of mycotoxin with a reversible ligand-grafted biosensing surface. Biosens Bioelectron 72:300–305
Tan Y, Wei X, Zhang Y, Wang P, Qiu B, Guo L, Lin Z, Yang H-H (2015) Exonuclease-Catalyzed Target Recycling Amplification and Immobilization-free Electrochemical Aptasensor. Anal Chem 87:11826–11831
Shen P, Li W, Liu Y, Ding Z, Deng Y, Zhu X, Jin Y, Li Y, Li J, Zheng T (2017) High-Throughput Low-Background G-Quadruplex Aptamer Chemiluminescence Assay for Ochratoxin A Using a Single Photonic Crystal Microsphere. Anal Chem 89:11862–11868
Chu X, Dou X, Liang R, Li M, Kong W, Yang X, Luo J, Yang M, Zhao M (2016) A self-assembly aptasensor based on thick-shell quantum dots for sensing of ochratoxin A. Nano 8:4127–4133
Yang J, Gao P, Liu Y, Li R, Ma H, Du B, Wei Q (2015) Label-free photoelectrochemical immunosensor for sensitive detection of Ochratoxin A. Biosens Bioelectron 64:13–18
Wang C, Qian J, Wang K, Yang X, Liu Q, Hao N, Wang C, Dong X, Huang X (2016) Colorimetric aptasensing of ochratoxin A using Au@Fe3O4 nanoparticles as signal indicator and magnetic separator. Biosens Bioelectron 77:1183–1191
Sun A-L, Zhang Y-F, Sun G-P, Wang X-N, Tang D (2017) Homogeneous electrochemical detection of ochratoxin A in foodstuff using aptamer–graphene oxide nanosheets and DNase I-based target recycling reaction. Biosens Bioelectron 89:659–665
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21505060), the Foundation of Jiangxi Educational Committee (GJJ150327), the Science Foundation of Jiangxi Province (20161BAB213073), Scientific Research Foundation of Jiangxi Normal University, the Open Project Program of Key Laboratory of Functional Small Organic Molecule, Ministry of Education, Jiangxi Normal University (No. KLFS-KF-201710), Outstanding Youth Funds of Jiangxi Normal University and the Advanced Research Fund of Quanzhou Normal University for Young Doctor (2016QBKJ03).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 4499 kb)
Rights and permissions
About this article
Cite this article
Tang, J., Huang, Y., Cheng, Y. et al. Two-dimensional MoS2 as a nano-binder for ssDNA: Ultrasensitive aptamer based amperometric detection of Ochratoxin A. Microchim Acta 185, 162 (2018). https://doi.org/10.1007/s00604-018-2706-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2706-9