Abstract
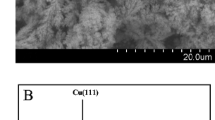
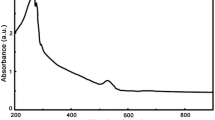
A non-enzymatic amperometric glucose is reported that is based on an glassy carbon electrode modified with a Cu-CuO nanowire (NW) composite. The morphology and the composition of the nanowire were characterized by scanning electron microscopy and X-ray diffraction, respectively. The modified electrode efficiently catalyzes the oxidation of glucose at less-positive potential (0.30 V) in 0.10 M NaOH solution in the absence of any enzymes or redox mediators. The sensor was successfully used for the amperometric sensing of glucose. Linear response was obtained over the concentration range from 0.1 to 12 mM. The common interfering agents ascorbic acid and uric acid do not interfere with the determination of glucose. The modified electrode features high sensitivity, low working potential, excellent stability, and fast amperometric sensing of glucose. Thus it is promising for the future development of non-enzymatic glucose sensors.






Similar content being viewed by others
References
Wen D, Liu Y, Yang GC, Dong SJ (2007) Electrochemistry of glucose oxidase immobilized on the carbon nanotube wrapped by polyelectrolyte. Electrochim Acta 52:5312
Wilson GS, Hu Y (2000) Enzyme-based biosensors for in vivo measurements. Chem Rev 100:2693
Wilson R, Turner APF (1992) Glucose oxidase: an ideal enzyme. Biosens Bioelectron 7:165
Zhou DM, Sun JJ, Chen HY, Fang HQ (1998) Electrochemical polymerization of toluidine blue and its application for the amperometric determination of β-d-glucose. Electrochim Acta 43:1803
Jena BK, Raj CR (2006) Enzyme-free amperometric sensing of glucose by using gold nnanoparticles. Chem Eur J 12:2702
Vassilyev YB, Khazova OA, Nikolaeva NN (1985) Kinetics and mechanism of glucose electrooxidation on different electrode-catalysts: Part I. Adsorption and oxidation on platinum. J Electroanal Chem 196:105
Li Y, Song YY, Yang C, Xia XH (2007) Hydrogen bubble dynamic template synthesis of porous gold for nonenzymatic electrochemical detection of glucose. Electrochem Commun 9:981
You CP, Li X, Zhang S, Kong JL, Zhao DY, Liu BH (2009) Electrochemistry and biosensing of glucose oxidase immobilized on Pt-dispersed mesoporous carbon. Microchimica Acta 167:109
Yi QF, Yu WQ (2009) Electrocatalytic activity of a novel titanium-supported nanoporous gold catalyst for glucose oxidation. Microchimica Acta 165:381
Luo P, Zhang F, Baldwin RP (1991) Comparison of metallic electrodes for constant-potential amperometric detection of carbohydrates, amino acids and related compounds in flow systems. Anal Chim Acta 244:169
Farrell ST, Breslin CB (2004) Oxidation and photo-induced oxidation of glucose at a polyaniline film modified by copper particles. Electrochim Acta 49:4497
Zhao J, Wang F, Yu J, Hu S (2006) Electro-oxidation of glucose at self-assembled monolayers incorporated by copper particles. Talanta 70:449
Aoun SB, Bang GS, Koga T, Nonaka Y, Sotomura T, Taniguchi I (2003) Electrocatalytic oxidation of sugars on silver-UPD single crystal gold electrodes in alkaline solutions. Electrochem Commun 5:317
Cao MH, Hu CW, Wang YH, Guo YH, Guo CX, Wang EB (2003) A controllable synthetic route to Cu, Cu2O, and CuO nanotubes and nanorods. Chem Commun 1884
Jiang XC, Herricks T, Xia YN (2002) CuO nanowires can be synthesized by heating copper substrates in air. Nano Lett 2:1333
Gao XP, Bao JL, Pan GL, Zhu HY, Huang PX, Wu F, Song DY (2004) Preparation and electrochemical performance of polycrystalline and single crystalline CuO nanorods as anode materials for Li ion battery. J Phys Chem B 108:5547
Liu Y, Chu Y, Li MY, Dong LH (2006) In situ synthesis and assembly of copper oxide nanocrystals on copper foil via a mild hydrothermal process. J Mater Chem 16:192
Zhang XJ, Wang GF, Zhang W, Hu NJ, Wu HQ, Fang B (2008) Seed-mediated growth method for epitaxial array of CuO nanowires on surface of Cu nanostructures and its application as a glucose Sensor. J Phys Chem C 112:8856
Liu H, Su X, Tian X, Huang Z, Song W, Zhao J (2006) Preparation and electrocatalytic performance of functionalized copper-based nanoparticles supported on the gold surface. Electroanalysis 18:2055
Kang X, Mai Z, Zou X, Cai P, Mo J (2007) A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal Biochem 363:143
Rong LQ, Yang C, Qian QY, Xia XH (2007) Study of the nonenzymatic glucose sensor based on highly dispersed Pt nanoparticles supported on carbon nanotubes. Talanta 72:819
Bard AJ, Faulkner LR (1982) Electrochemical methods fundamentals and applications. Wiley, New York
Casella IG, Gatta M, Guascito MR, Cataldi TRI (1997) Highly-dispersed copper microparticles on the active gold substrate as an amperometric sensor for glucose. Anal Chim Acta 357:63
Liu BH, Hu RQ, Deng JQ (1997) Characterization of immobilization of an enzyme in a modified Y zeolite matrix and its application to an amperometric glucose biosensor. Anal Chem 69:2343
Zhuang ZJ, Su XD, Yuan HY, Sun Q, Xiao D, Choi MMF (2008) An improved sensitivity non-enzymatic glucose sensor based on a CuO nanowire modified Cu electrode. Analyst 133:126
Zhao H, Zhang YZ, Yuan ZB (2001) Study on the electrochemical behavior of dopamine with poly (sulfosalicylic acid) modified glassy carbon electrode. Anal Chim Acta 441:117
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.20675001, 20901003), Anhui Key Laboratory of Controllable Chemistry Reaction & Material Chemical Engineering, the Young Teacher Program of Anhui Normal University (2009xqnzc19), and the Natural Science Foundation of Educational Department of Anhui Province (No. KJ2008B167, No. KJ2009B013Z).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, G., Wei, Y., Zhang, W. et al. Enzyme-free amperometric sensing of glucose using Cu-CuO nanowire composites. Microchim Acta 168, 87–92 (2010). https://doi.org/10.1007/s00604-009-0260-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-009-0260-1