Abstract
Aims
Diabetes is associated with an excess release of neutrophil extracellular traps (NETs) and an enhanced NETosis, a neutrophil cell death programme instrumental to anti-microbial defences, but also involved in tissue damage. We herein investigated whether the antidiabetic drug metformin protects against NETosis.
Methods
We measured NET components in the plasma of patients with pre-diabetes who were randomized to receive metformin or placebo for 2 months. To control for the effect on glucose, we also measured NET components in the plasma of patients with type 2 diabetes before and after treatment with insulin or dapagliflozin. In vitro, we used static and dynamic imaging with advanced live confocal two-photon microscopy to evaluate the effects of metformin on cellular events during NETosis. We examined putative molecular mechanisms by monitoring chromatin decondensation and DNA release in vitro.
Results
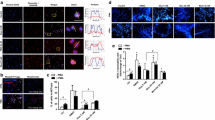
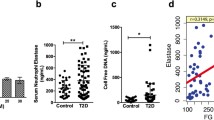
Metformin, as compared to placebo, significantly reduced the concentrations of NET components elastase, proteinase-3, histones and double strand DNA, whereas glucose control with insulin or dapagliflozin exerted no significant effect. In vitro, metformin prevented pathologic changes in nuclear dynamics and DNA release, resulting in a blunted NETosis in response to phorbol myristate acetate and calcium influx. Metformin prevented membrane translocation of PKC-βII and activation of NADPH oxidase in neutrophils, both of which diminished the NETosis response.
Conclusions
Metformin treatment reduced the concentrations of NET components independently from glucose control. This effect was reproducible in vitro and was related to the inhibitory effect exerted by metformin on the PKC-NADPH oxidase pathway.




Similar content being viewed by others
References
Wellen KE, Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Investig 115:1111–1119
Cimini FA, Barchetta I, Porzia A et al (2017) Circulating IL-8 levels are increased in patients with type 2 diabetes and associated with worse inflammatory and cardiometabolic profile. Acta Diabetol 54:961–967
Mocsai A (2013) Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 210:1283–1299
Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T (2011) Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ 18:581–588
Douda DN, Khan MA, Grasemann H, Palaniyar N (2015) SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci USA 112:2817–2822
Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V (2014) A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep 8:883–896
Leshner M, Wang S, Lewis C et al (2012) PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol 3:307
Brinkmann V, Reichard U, Goosmann C et al (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–1535
Villanueva E, Yalavarthi S, Berthier CC et al (2011) Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 187:538–552
Martinod K, Fuchs TA, Zitomersky NL et al (2015) PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 125:1948–1956
Menegazzo L, Ciciliot S, Poncina N et al (2015) NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol 52:497–503
Fadini GP, Menegazzo L, Rigato M et al (2016) NETosis delays diabetic wound healing in mice and humans. Diabetes 65:1061–1071
Wong SL, Demers M, Martinod K et al (2015) Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 21:815–819
Batchuluun B, Inoguchi T, Sonoda N et al (2014) Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis 232:156–164
Gallo A, Ceolotto G, Pinton P et al (2005) Metformin prevents glucose-induced protein kinase C-beta2 activation in human umbilical vein endothelial cells through an antioxidant mechanism. Diabetes 54:1123–1131
Wu N, Shen H, Wang Y et al (2017) Role of the PKCbetaII/JNK signaling pathway in acute glucose fluctuation-induced apoptosis of rat vascular endothelial cells. Acta Diabetol 54:727–736
Peixoto LG, Teixeira RR, Vilela DD et al (2017) Metformin attenuates the TLR4 inflammatory pathway in skeletal muscle of diabetic rats. Acta Diabetol 54:943–951
Rena G, Lang CC (2018) Repurposing metformin for cardiovascular disease. Circulation 137:422–424
de Kreutzenberg SV, Ceolotto G, Cattelan A et al (2015) Metformin improves putative longevity effectors in peripheral mononuclear cells from subjects with prediabetes. A randomized controlled trial. Nutr Metab Cardiovasc Dis 25:686–693
Fadini GP, de Kreutzenberg SV, Mariano V et al (2011) Optimized glycaemic control achieved with add-on basal insulin therapy improves indexes of endothelial damage and regeneration in type 2 diabetic patients with macroangiopathy: a randomized crossover trial comparing detemir versus glargine. Diabetes Obes Metab 13:718–725
Fadini GP, Bonora BM, Zatti G et al (2017) Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovasc Diabetol 16:42
Raad H, Paclet MH, Boussetta T et al (2009) Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. FASEB J 23:1011–1022
Kenny EF, Herzig A, Kruger R et al (2017) Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 6:e24437
Carestia A, Frechtel G, Cerrone G et al (2016) NETosis before and after hyperglycemic control in type 2 diabetes mellitus patients. PLoS ONE 11:e0168647
Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J (2016) Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell 164:57–68
Tay HM, Dalan R, Li KHH, Boehm BO, Hou HW (2018) A novel microdevice for rapid neutrophil purification and phenotyping in type 2 diabetes mellitus. Small 14(6). https://doi.org/10.1002/smll.201702832
Wang H, Li T, Chen S, Gu Y, Ye S (2015) Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol 67:3190–3200
Wu L, Zhou B, Oshiro-Rapley N et al (2016) An ancient, unified mechanism for metformin growth inhibition in C. elegans and cancer. Cell 167(1705–1718):e1713
Amulic B, Knackstedt SL, Abu Abed U et al (2017) Cell-cycle proteins control production of neutrophil extracellular traps. Dev Cell 43(449–462):e445
Funding
The study was supported by grants from: the Italian Ministry of Health grant to MA (RF-2013-02358024); the University of Padova 2011 Strategic Project DYCENDI Grant to AA; The University of Padova STARS Grant to GPF; the European Foundation for the Study of Diabetes (EFSD)/Lilly Grant 2016 to GPF; an AstraZeneca Grant to AA. The sponsors had not role in study design, data analysis and interpretation and decision to publish.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Ethical standard
The study was approved by the local ethical committee and carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008. All subjects provided written informed consent.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Managed by Antonio Secchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Menegazzo, L., Scattolini, V., Cappellari, R. et al. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol 55, 593–601 (2018). https://doi.org/10.1007/s00592-018-1129-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1129-8