Abstract
Purpose
This study aimed to compare the effect of needle puncture and chondroitinase ABC (ChABC) injection on inducing intervertebral disc (IVD) degeneration (IVDD) in rabbits.
Methods
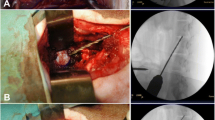
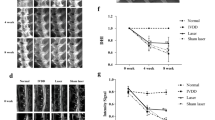
Sixteen New Zealand white rabbits were used in this study. Briefly, the rabbits were divided into four groups. In the annulus fibrosis (AF) needle puncture group, a 16-G needle was used to puncture the L5-6 and L6-7 IVDs, while in the sham group, these IVDs were not punctured. In the ChABC group, 30 μL 0.5 Unit/mL ChABC was injected into L5-6 and L6-7 IVDs using a 26-G needle, while in the vehicle group, these IVDs were injected with 30 μL phosphate-buffered saline (PBS). X-ray and MRI scans were performed at the 4th, 12th and 16th weeks postoperatively. Histological, immunohistochemical and biochemical analyses were performed at the 16th week postoperatively.
Results
Both needle puncture and ChABC successfully established IVDD in rabbits at 4th, 12th and 16th weeks, confirmed by X-ray and MRI scan. The progression of IVDD went in a time-dependent manner. The IVDD in the ChABC group was less severe than in the needle puncture group throughout the study. Aggrecan and type II collagen significantly decreased, while tumor necrosis factor-α and superoxide dismutase 2 increased in the needle puncture and ChABC groups, compared with the sham and PBS groups.
Conclusions
Both AF needle puncture and ChABC injection can successfully induce IVDD in rabbits. Compared with ChABC injection, AF needle puncture can induce more severe IVDD.





Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- AB:
-
Alcian blue
- ADAMTs:
-
A disintegrin and metalloproteinase with thrombospondin motifs
- AF:
-
Annulus fibrosis
- ChABC:
-
Chondroitinase ABC
- COL2:
-
Type II collagen
- DHI:
-
Disc height index
- ECM:
-
Extracellular matrix
- H&E:
-
Hematoxylin and eosin
- IL-1:
-
Interleukin-1
- IL-17:
-
Interleukin-17
- IQR:
-
Interquartile range
- IVD:
-
Intervertebral disc
- IVDD:
-
Intervertebral disc degeneration
- LBP:
-
Low back pain
- MMPs:
-
Matrix metalloproteinases
- NP:
-
Nucleus pulposus
- PBS:
-
Phosphate-buffered saline
- ROI:
-
Region of interest
- SO/FG:
-
Safranin O/Fast green
- SOD2:
-
Superoxide dismutase 2
- TNF-α:
-
Tumor necrosis factor-α
References
Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, Williams G, Smith E, Vos T, Barendregt J, Murray C, Burstein R, Buchbinder R (2014) The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 73:968–974. https://doi.org/10.1136/annrheumdis-2013-204428
Katz JN (2006) Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am 88(Suppl 2):21–24. https://doi.org/10.2106/jbjs.E.01273
Anderson DG, Tannoury C (2005) Molecular pathogenic factors in symptomatic disc degeneration. Spine J 5:260s–266s. https://doi.org/10.1016/j.spinee.205.02.010
Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A (2000) Low back pain in relation to lumbar disc degeneration. Spine 25:487–492. https://doi.org/10.1097/00007632-200002150-00016
Aguiar DJ, Johnson SL, Oegema TR (1999) Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res 246:129–137. https://doi.org/10.1006/excr.1998.4287
Urban JP, Roberts S (2003) Degeneration of the intervertebral disc. Arthritis Res Ther 5:120–130. https://doi.org/10.1186/ar629
Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S (1996) T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med 183:2593–2603. https://doi.org/10.1084/jem.183.6.2593
Le Maitre CL, Hoyland JA, Freemont AJ (2007) Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther 9:R77. https://doi.org/10.1186/ar2275
Yang S, Zhang F, Ma J, Ding W (2020) Intervertebral disc ageing and degeneration: The antiapoptotic effect of oestrogen. Ageing Res Rev 57:100978. https://doi.org/10.1016/j.arr.2019.100978
Smith LJ, Chiaro JA, Nerurkar NL, Cortes DH, Horava SD, Hebela NM, Mauck RL, Dodge GR, Elliott DM (2011) Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine challenge following long-term agarose culture. Eur Cell Mater 22:291–301. https://doi.org/10.22203/ecm.v022a22
Pockert AJ, Richardson SM, Le Maitre CL, Lyon M, Deakin JA, Buttle DJ, Freemont AJ, Hoyland JA (2009) Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum 60:482–491. https://doi.org/10.1002/art.24291
Lee DC, Adams CS, Albert TJ, Shapiro IM, Evans SM, Koch CJ (2007) In situ oxygen utilization in the rat intervertebral disc. J Anat 210:294–303. https://doi.org/10.1155/2020/6660429
Qin R, Dai S, Zhang X, Liu H, Zhou B, Zhou P, Hu C (2020) Danshen attenuates intervertebral disc degeneration via antioxidation in SD rats. Oxid Med Cell Longev 2020:6660429. https://doi.org/10.1155/2020/6660429
Suzuki S, Fujita N, Hosogane N, Watanabe K, Ishii K, Toyama Y, Takubo K, Horiuchi K, Miyamoto T, Nakamura M, Matsumoto M (2015) Excessive reactive oxygen species are therapeutic targets for intervertebral disc degeneration. Arthritis Res Ther 17:316. https://doi.org/10.1186/s13075-015-0834-8
Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D (2015) Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cells Mater 30:89–103. https://doi.org/10.22203/eCM.v030a07
Lee CS, Hwang CJ, Lee SW, Ahn YJ, Kim YT, Lee DH, Lee MY (2009) Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J 18:1637–1643. https://doi.org/10.1007/s00586-009-1060-3
van Uden S, Silva-Correia J, Oliveira JM, Reis RL (2017) Current strategies for treatment of intervertebral disc degeneration: substitution and regeneration possibilities. Biomater Res 21:22. https://doi.org/10.1186/s40824-017-0106-6
Sloan SR Jr, Lintz M, Hussain I, Hartl R, Bonassar LJ (2018) Biologic annulus fibrosus repair: a review of preclinical In Vivo investigations. Tissue Eng Part B Rev 24:179–190. https://doi.org/10.1089/ten.TEB.2017.0351
Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I, Wilke HJ (2008) Are animal models useful for studying human disc disorders/degeneration? Eur Spine J 17:2–19. https://doi.org/10.1007/s00586-007-0414-y
Singh K, Masuda K, An HS (2005) Animal models for human disc degeneration. Spine J 5:267s–279s. https://doi.org/10.1016/j.spinee.2005.02.016
Kim KS, Yoon ST, Li J, Park JS, Hutton WC (2005) Disc degeneration in the rabbit: a biochemical and radiological comparison between four disc injury models. Spine 30:33–37. https://doi.org/10.1097/01.brs.0000149191.02304.9b
Kroeber MW, Unglaub F, Wang H, Schmid C, Thomsen M, Nerlich A, Richter W (2002) New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine 27:2684–2690. https://doi.org/10.1097/00007632-200212010-00007
Lotz JC (2004) Animal models of intervertebral disc degeneration: lessons learned. Spine 29:2742–2750. https://doi.org/10.1097/01.brs.0000146498.04628.f9
Sun F, Qu JN, Zhang YG (2013) Animal models of disc degeneration and major genetic strategies. Pain Physician 16:E267-275
Moss IL, Zhang Y, Shi P, Chee A, Piel MJ, An HS (2013) Retroperitoneal approach to the intervertebral disc for the annular puncture model of intervertebral disc degeneration in the rabbit. Spine J 13:229–234. https://doi.org/10.1016/j.spinee.2012.02.028
Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, An HS (2005) A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 30:5–14. https://doi.org/10.1097/01.brs.0000148152.04401.20
Ando T, Kato F, Mimatsu K, Iwata H (1995) Effects of chondroitinase ABC on degenerative intervertebral discs. Clin Orthop Relat Res 214–221
Liu S, Yang SD, Huo XW, Yang DL, Ma L, Ding WY (2018) 17β-Estradiol inhibits intervertebral disc degeneration by down-regulating MMP-3 and MMP-13 and up-regulating type II collagen in a rat model. Artif Cells Nanomed Biotechnol 46:182–191. https://doi.org/10.1080/21691401.2018.1453826
Leckie SK, Sowa GA, Bechara BP, Hartman RA, Coelho JP, Witt WT, Dong QD, Bowman BW, Bell KM, Vo NV, Kramer BC, Kang JD (2013) Injection of human umbilical tissue-derived cells into the nucleus pulposus alters the course of intervertebral disc degeneration in vivo. Spine J 13:263–272. https://doi.org/10.1016/j.spinee.2012.12.004
Qian J, Ge J, Yan Q, Wu C, Yang H, Zou J (2019) Selection of the optimal puncture needle for induction of a rat intervertebral disc degeneration model. Pain Physician 22:353–360
Elliott DM, Yerramalli CS, Beckstein JC, Boxberger JI, Johannessen W, Vresilovic EJ (2008) The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine 33:588–596. https://doi.org/10.1097/BRS.0b013e318166e0a2
Hoogendoorn RJ, Wuisman PI, Smit TH, Everts VE, Helder MN (2007) Experimental intervertebral disc degeneration induced by chondroitinase ABC in the goat. Spine 32:1816–1825. https://doi.org/10.1097/BRS.0b013e31811ebac5
Borem R, Walters J, Madeline A, Madeline L, Gill S, Easley J, Mercuri J (2021) Characterization of chondroitinase-induced lumbar intervertebral disc degeneration in a sheep model intended for assessing biomaterials. J Biomed Mater Res A 109:1232–1246. https://doi.org/10.1002/jbm.a.37117
Kato F, Iwata H, Mimatsu K, Miura T (1990) Experimental chemonucleolysis with chondroitinase ABC. Clin Orthop Relat Res 235:301–308
Park JS, Ahn JI (1995) The effect of chondroitinase ABC on rabbit intervertebral disc. Radiological, histological and electron microscopic findings. Int Orthop 19:103–109. https://doi.org/10.1007/bf00179970
Takahashi T, Kurihara H, Nakajima S, Kato T, Matsuzaka S, Sekiguchi T, Onaya M, Miyauchi S, Mizuno S, Horie K, Fujita Y, Hirose T (1996) Chemonucleolytic effects of chondroitinase ABC on normal rabbit intervertebral discs. Course of action up to 10 days postinjection and minimum effective dose. Spine 21:2405–2411. https://doi.org/10.1097/00007632-199611010-00001
Leung VY, Chan D, Cheung KM (2006) Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction. Eur Spine J 3:S406-413. https://doi.org/10.1007/s00586-006-0183-z
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26:1873–1878. https://doi.org/10.1097/00007632-200109010-00011
Sobajima S, Kompel JF, Kim JS, Wallach CJ, Robertson DD, Vogt MT, Kang JD, Gilbertson LG (2005) A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine 30:15–24. https://doi.org/10.1097/01.brs.0000148048.15348.9b
Daly C, Ghosh P, Jenkin G, Oehme D, Goldschlager T (2016) A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int 2016:5952165. https://doi.org/10.1155/2016/5952165
Sasaki M, Takahashi T, Miyahara K, Hirosea T (2001) Effects of chondroitinase ABC on intradiscal pressure in sheep: an in vivo study. Spine 26:463–468. https://doi.org/10.1097/00007632-200103010-00008
Xi Y, Kong J, Liu Y, Wang Z, Ren S, Diao Z, Hu Y (2013) Minimally invasive induction of an early lumbar disc degeneration model in rhesus monkeys. Spine 38:E579-586. https://doi.org/10.1097/BRS.0b013e31828b695b
Lei T, Zhang Y, Zhou Q, Luo X, Tang K, Chen R, Yu C, Quan Z (2017) A novel approach for the annulus needle puncture model of intervertebral disc degeneration in rabbits. Am J Transl Res 9:900–909
Grunhagen T, Shirazi-Adl A, Fairbank JC, Urban JP (2011) Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am 42:465–477. https://doi.org/10.1016/j.ocl.2011.07.010
Guehring T, Wilde G, Sumner M, Grünhagen T, Karney GB, Tirlapur UK, Urban JP (2009) Notochordal intervertebral disc cells: sensitivity to nutrient deprivation. Arthritis Rheum 60:1026–1034. https://doi.org/10.1002/art.24407
Acknowledgements
We would like to give special thanks to Mr. Samuel Rudd, who is from The University of Queensland, Australia, for checking and editing the language used in this paper.
Funding
This study was supported by Natural Science Foundation of Hebei Province (No. H2021108006).
Author information
Authors and Affiliations
Contributions
SY, ZW, WD and WZ: were responsible for study design. SY, ZS, DJ, YH, YZ and KY: contributed to surgical implementation and sample processing. SY, JM, YH and KY: were responsible for data analysis. KY: drafted the manuscript. SY and KY: edited and revised the manuscript. All authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
These authors have no conflict of interest to declare.
Ethical approval
All research protocols have been approved by the Animal Care and Use Committee of Orthopedic Hospital of Xingtai.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, K., Song, Z., Jia, D. et al. Comparisons between needle puncture and chondroitinase ABC to induce intervertebral disc degeneration in rabbits. Eur Spine J 31, 2788–2800 (2022). https://doi.org/10.1007/s00586-022-07287-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07287-8