Abstract
Purpose
Platelet concentrates in spine fusion gained increasing popularity among spine surgeons. They avoid morbidity of bone harvest and promise good union rates without additional device-related adverse events. Therefore, they seem to be a safe and effective alternative to common bone substitutes. This meta-analysis assesses the available evidence for union rate and overall complications with the use of platelet concentrates in spine fusion.
Methods
We conducted an online search for relevant controlled trials and extracted data on union rates, complications, and revision rates. These data were synthesized in a meta-analysis using fixed-effects odds ratios (OR). To assess covariates, meta-regression was performed as well.
Results
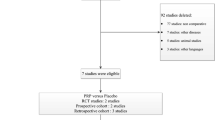
Our search produced 166 results, ten of which were eligible for inclusion. These studies report on a total of 763 patients (328 experimental, 435 controls) with a mean age of 50.3 ± 7.5 years. Mean follow-up was 1.9 ± 0.0.4 years. With the use of platelet concentrates, union rate decreased significantly, OR 0.53 (95 % CI 0.35–0.79, p = 0.002), compared with the control group. There was no statistically significant difference in complication rates OR 1.34 (95 % CI 0.62–2.90, p = 0.46) or in revision rates OR 3.0 (95 % CI 0.90–10.00, p = 0.74). Meta-regression showed no statistically significant influence of randomization, Jadad score, or assessment of fusion.
Conclusion
The use of platelet concentrates in spine fusion shows significantly decreased union rates compared with the control group. However, complication and revision rates were not significantly increased. The current data do not recommend the use of platelet concentrate in spine fusion.




Similar content being viewed by others
References
Schwartz CE, Martha JF, Kowalski P et al (2009) Prospective evaluation of chronic pain associated with posterior autologous iliac crest bone graft harvest and its effect on postoperative outcome. Health Qual Life Outcomes 7(1):49–56
Müller MA, Mehrkens A, Zürcher R et al (2014) Effectiveness of the addition of Lidocaine to a hemostatic, bioresorbable putty in the treatment of iliac crest donor site pain. BMC Musculoskeletal Disord 15(1):415
Rihn JA, Kirkpatrick K, Albert TJ (2010) Graft options in posterolateral and posterior interbody lumbar fusion. Spine 35(17):1629–1639
Fischer CR, Cassilly R, Cantor W et al (2013) A systematic review of comparative studies on bone graft alternatives for common spine fusion procedures. Eur Spine J 22(6):1423–1435
Fu R, Selph S, McDonagh M et al (2013) Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med 158(12):890–902
Simmonds MC, Brown JVE, Heirs MK et al (2013) Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med 158(12):877–889
Vavken J, Mameghani A, Vavken P, Schaeren S (2015) Complications and cancer rates in spine fusion with recombinant human bone morphogenetic protein-2 (rhBMP-2). Eur Spine J :1–11
Landi A, Tarantino R, Marotta N, Ruggeri AG (2011) The use of platelet gel in postero-lateral fusion: preliminary results in a series of 14 cases.Eur Spine J 20(Suppl 1):S61–S67
Hustedt JW, Blizzard DJ (2014) The controversy surrounding bone morphogenetic proteins in the spine: a review of current research. Yale J Biol Med 87(4):549–561
Vavken P, Sadoghi P, Murray MM (2011) The effect of platelet concentrates on graft maturation and graft-bone interface healing in anterior cruciate ligament reconstruction in human patients: a systematic review of controlled trials. YJARS 27(11):1573–1583
Sadoghi P, Lohberger B, Aigner B et al (2013) Effect of platelet-rich plasma on the biologic activity of the human rotator-cuff fibroblasts: a controlled in vitro study. J Orthop Res 31(8):1249–1253
Malhotra A, Pelletier MH, Yu Y, Walsh WR (2012) Can platelet-rich plasma (PRP) improve bone healing? A comparison between the theory and experimental outcomes. Arch Orthop Trauma Surg 133(2):153–165
Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62(4):489–496
Tarantino R, Donnarumma P, Mancarella C et al (2014) Posterolateral arthrodesis in lumbar spine surgery using autologous platelet-rich plasma and cancellous bone substitute: an osteoinductive and osteoconductive effect. Global Spine J 04(03):137–142
Vaccaro AR, Sharan AD, Tuan RS et al (2001) The use of biologic materials in spinal fusion. Orthopedics 24(2):191–197 (quiz 198–199)
Moher D, Altman DG, Liberati A, Tetzlaff J (2011) PRISMA Statement. Epidemiology 22(1):128–133
Moher D, Cook DJ, Eastwood S et al (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet 354(9193):1896–1900
Jadad AR, Enkin MW (2008) Randomized controlled trials. Wiley p 1
Egger M, Smith GD, Altman D (2001) Systematic reviews in health care. BMJ Books, p 1
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269
Hee HT, Majd ME, Holt RT, Myers L (2003) Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J 12(4):400–407
Weiner BK, Walker M (2003) Efficacy of autologous growth factors in lumbar intertransverse fusions. Spine 28(17):1968–1970
Castro FP (2004) Role of Activated Growth Factors in Lumbar Spinal Fusions. J Spinal Disord Techn 17(5):380–384
Carreon LY, Glassman SD, Anekstein Y, Puno RM (2005) Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine 30(9):1–4
Jenis LG, Banco RJ, Kwon B (2006) A prospective study of autologous growth factors (AGF) in lumbar interbody fusion. Spine J 6(1):14–20
Feiz-Erfan I, Harrigan M, Sonntag V, Harrington TR (2007) Effect of autologous platelet gel on early and late graft fusion in anterior cervical spine surgery. J Neurosurg Spine 7(5):496–502
Tsai C-H, Hsu H-C, Chen Y-J et al (2009) Using the growth factors-enriched platelet glue in spinal fusion and its efficiency. J Spinal Disord Tech 22(4):246–25028
Hartmann EK, Heintel T, Morrison RH, Weckbach A (2009) Influence of platelet-rich plasma on the anterior fusion in spinal injuries: a qualitative and quantitative analysis using computer tomography. Arch Orthop Trauma Surg 130(7):909–914
Acebal-Cortina G, Suárez-Suárez MA, García-Menéndez C et al (2011) Evaluation of autologous platelet concentrate for intertransverse lumbar fusion. Eur Spine J 20(S3):361–366
Sys J, Weyler J, Van Der Zijden T et al (2011) Platelet-rich plasma in mono-segmental posterior lumbar interbody fusion. Eur Spine J 20(10):1650–1657
Gertzbein SD, Betz R, Clements D et al (1996) Semirigid instrumentation in the management of lumbar spinal conditions combined with circumferential fusion. A multicenter study. Spine 21(16):1918–1925 (discussion 1925–6)
Boden SD, Zdeblick TA, Sandhu HS, Heim SE (2000) The use of rhBMP-2 in interbody fusion cages: definitive evidence of osteoinduction in humans: a preliminary report. Spine 25(3):376–381
Boden SD, Kang J, Sandhu H, Heller JG (2002) Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial 2002 volvo award in clinical studies. Spine 27(23):2662–2673
Ong KL, Villarraga ML, Lau E et al (2010) Off-label use of bone morphogenetic proteins in the united states using administrative data. Spine 35(19):1794–1800
Carragee EJ, Mitsunaga KA, Hurwitz EL, Scuderi GJ (2011) Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP-2: a cohort controlled study. Spine 11(6):511–516
Carragee EJ, Hurwitz EL, Weiner BK (2011) A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine 11(6):471–491
Singh K, Nandyala SV, Marquez-Lara A et al (2013) Clinical sequelae after rhBMP-2 use in a minimally invasive transforaminal lumbar interbody fusion. Spine 13(9):1118–1125
Choi B-H, Im C-J, Huh J-Y et al (2004) Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg 33(1):56–59
Butterfield KJ, Bennett J, Gronowicz G, Adams D (2005) Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg 63(3):370–376
Sarkar MR, Augat P, Shefelbine SJ et al (2006) Bone formation in a long bone defect model using a platelet-rich plasmaloaded collagen scaffold. Biomaterials 2006(27):1817–182341
Schaaf H, Streckbein P, Lendeckel S et al (2008) Sinus lift augmentation using autogenous bone grafts and platelet-rich plasma: radiographic results. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106(5):673–678
Li H, Zou X, Xue Q et al (2004) Anterior lumbar interbody fusion with carbon fiber cage loaded with bioceramics and platelet-rich plasma. An experimental study on pigs. Eur Spine J 13(4):354–358
Veillette CJH, McKee MD (2007) Growth factors—BMPs, DBMs, and buffy coat products: are there any proven differences amongst them? Injury 38(1):S38–S48
BK050016—FDA marketing authorization letter for the GPS II Platelet Concentrate Separation Kit w/Plasma Concentrator. 2005 Mar 22. http://www.fda.govcberseltrkL.htm [cited 2007 May 18]
BK060004—FDA Marketing Authorization letter for the Smith and Nephew Platelet Rich Concentrate (PRC). http://www.fda.govcberseltrkL.htm [cited 2015 Feb 19]
Lowery GL, Kulkarni S, Pennisi AE (1999) Use of autologous growth factors in lumbar spinal fusion. Bone 25(2):47S–50S
Bose B, Michael A Balzarini. 2002. Bone graft gel: autologous growth factors used with autograft bone for lumbar spine fusions. Adv Therap 19(4):1–6
Weibrich G, Kleis WK, Kunz-Kostomanolakis M et al (2001) Correlation of platelet concentration in platelet-rich plasma to the extraction method, age, sex, and platelet count of the donor. Int J Oral Maxillofac Implants 16(5):693–699
Weibrich G, Kleis WKG, Hafner G, Hitzler WE (2002) Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg 30(2):97–102
Mazzocca AD, McCarthy MBR, Chowaniec DM et al (2012) Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am 94(4):308–316
Acknowledgments
No funding was obtained for this study
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vavken, J., Vavken, P., Mameghani, A. et al. Platelet concentrates in spine fusion: meta-analysis of union rates and complications in controlled trials. Eur Spine J 25, 1474–1483 (2016). https://doi.org/10.1007/s00586-015-4193-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-4193-6