Abstract
Arbuscular mycorrhizal fungi (AMF) have been implicated in non-native plant invasion success and persistence. However, few studies have identified the AMF species associating directly with plant invaders, or how these associations differ from those of native plant species. Identifying changes to the AMF community due to plant invasion could yield key plant–AMF interactions necessary for the restoration of native plant communities. This research compared AMF associating with coexisting Bromus tectorum, an invasive annual grass, and Artemisia tridentata, the dominant native shrub in western North America. At three sites, soil and root samples from Bromus and Artemisia were collected. Sporulation was induced using trap cultures, and spores were identified using morphological characteristics. DNA was extracted from root and soil subsamples and amplified. Sequences obtained were aligned and analyzed to compare diversity, composition, and phylogenetic distance between hosts and sites. Richness of AMF species associated with Artemisia in cultures was higher than AMF species associated with Bromus. Gamma diversity was similar and beta diversity was higher in AMF associated with Bromus compared to Artemisia. AMF community composition differed between hosts in both cultures and roots. Two AMF species (Archaeospora trappei and Viscospora viscosum) associated more frequently with Artemisia than Bromus across multiple sites. AMF communities in Bromus roots were more phylogenetically dispersed than in Artemisia roots, indicating a greater competition for resources within the invasive grass. Bromus associated with an AMF community that differed from Artemisia in a number of ways, and these changes could restrict native plant establishment.




Similar content being viewed by others
References
Allen EB (1984) VA mycorrhizae and colonizing annuals: implications for growth, competition, and succession. In: Williams SE, Allen MF (eds) VA mycorrhizae and reclamation of arid and semi-arid lands. Wyoming Agricultural Experiment Station Scientific Report SA1261. University of Wyoming, Laramie, pp 42–52
Allen EB, Allen MF, Helm DJ, Trappe JM, Molina R, Rincon E (1995) Patterns and regulation of mycorrhizal plant and fungal diversity. Plant Soil 170:47–62
Al-Qawari AA (2002) Relationships among nitrogen availability, vesicular-arbuscular mycorrhizae, and Bromus tectorum in disturbed rangeland sites in Colorado. Colorado State University, Dissertation
Azcón-Aguilar C, Palenzuela J, Roldán A, Bautista S, Vallejo R, Barea JM (2003) Analysis of the mycorrhizal potential in the rhizosphere of representative plant species from desertification-threatened Mediterranean shrublands. Appl Soil Ecol 22(2):9–37
Baker WL (2006) Fire and restoration of sagebrush ecosystems. Wildlife Soc B 34:177–185
Bechtold HA, Inouye RS (2007) Distribution of carbon and nitrogen in sagebrush steppe after six years of nitrogen addition and shrub removal. J Arid Environ 71:122–132
Blaszkowski J, Kovacs GM, Balazs TK, Orlowska E, Sadravi M, Wubet T, Buscot F (2010a) Glomus africanum and G. iranicum, two new species of arbuscular mycorrhizal fungi (Glomeromycota). Mycologia 102:1450–1462
Blaszkowski J, Wubet T, Harikumar VS, Ryszka P, Buscot F (2010b) Glomus indicum, a new arbuscular mycorrhizal fungus. Botany 88:132–143
Brown D, Asplund O, McMahon VA (1975) Phenolic constituents of Artemisia tridentata ssp. vaseyana. Phytochemistry 14:1083–1084
Burke IC, Reiners WA, Sturges DL, Matson PA (1987) Herbicide treatment effects on properties of mountain Artemisia soils after fourteen years. Soil Sci Soc Am J 51:1337–1343
Busby RR, Gebhart DL, Stromberger ME, Meiman PJ, Paschke MW (2011) Early seral plant species’ interactions with an arbuscular mycorrhizal fungi community are highly variable. Appl Soil Ecol 48:257–262
Busby RR, Paschke MW, Stromberger ME, Gebhart DL (2012) Seasonal variation in arbuscular mycorrhizal fungi root colonization of cheatgrass (Bromus tectorum), an invasive winter annual. J Ecosyst Ecography. doi:10.4172/2157-7625.S8-001
Callaway RM, Thelen GC, Rodriguez A, Holben WE (2004) Soil biota and exotic plant invasion. Nature 427:731–733
Callaway RM, Cipollini D, Barto K, Thelen GC, Hallett SG, Prati D, Stinson K, Klironomos J (2008) Novel weapons: invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 89:1043–1055
D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass/fire cycle, and global change. Ann Rev Ecol Syst 23:63–87
Daniels BA, Skipper HD (1982) Methods for the recovery and quantitative estimation of propagules from soil. In: Schenk NC (ed) Methods and principles of mycorrhizal research. American Phytopathological Society Press, St. Paul, pp 29–35
Eom AH, Wilson GWT, Hartnett DC (2001) Effects of ungulate grazers on arbuscular mycorrhizal symbioses and fungal community structure in tallgrass prairie. Mycologia 93:233–242
Friar EA (2005) Isolation of DNA from plants with large amounts of secondary metabolites. Method Enzymol 395:3–14
Gustafson DJ, Casper BB (2006) Differential host plant performance as a function of soil arbuscular mycorrhizal fungal communities: experimentally manipulating co-occurring Glomus species. Plant Ecol 183:257–263
Harner MJ, Mummey DL, Stanford JA, Rillig MC (2010) Arbuscular mycorrhizal fungi enhance spotted knapweed growth across a riparian chronosequence. Biol Invasions 12:1481–1490
Hausmann NT, Hawkes CV (2009) Plant neighborhood control of arbuscular mycorrhizal community composition. New Phytol 183:1188–1200
Hausmann NT, Hawkes CV (2010) Order of plant host establishment alters composition of arbuscular mycorrhizal communities. Ecology 91:2333–2343
Hawkes CV, Belnap J, D’Antonio C, Firestone MK (2006) Arbuscular mycorrhizal assemblages in native plant roots change in the presence of invasive exotic grasses. Plant Soil 281:369–380
Helgason T, Daniell TJ, Husband R, Fitter AH, Young JPW (1998) Ploughing up the wood-wide web? Nature 394:431
Huber T, Faulkner G, Hugenholtz P (2004) Bellerophon; a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317–2319
Jassbi AR, Zamanizadehnajari S, Baldwin IT (2010) Phytotoxic volatiles in the roots and shoots of Artemisia tridentata as detected by headspace solid-phase microextraction and gas chromatographic-mass spectrometry analysis. J Chem Ecol 36:1398–1407
Klemmedson JO, Smith JG (1964) Bromus (Bromus tectorum L.). Bot Rev 30:226–262
Knapp PA (1996) Bromus (Bromus tectorum L.) dominance in the Great Basin Desert: history, persistence, and influences to human activities. Global Env Change 6:37–52
Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res 92:486–488
Koske RE, Tessier B (1983) A convenient, permanent slide mounting medium. Mycol Soc Am Newsl 34:59
Lee J-K, Tae M-S, Eom A-H, Lee SS (2003) Restriction analyses of PCR amplified partial SSU ribosomal DNA to distinguish arbuscular mycorrhizal fungi from other fungi colonizing plant roots. Mycobiology 31:68–73
Lindsey DL (1984) The role of vesicular-arbuscular mycorrhizae in shrub establishment. In: Williams SE, Allen MF (eds) VA mycorrhizae and reclamation of arid and semi-arid lands. Wyoming Agricultural Experiment Station Scientific Report SA1261. University of Wyoming, Laramie, pp 53–68
Mack RN (1989) Temperate grasslands vulnerable to plant invasions: characteristics and consequences. In: Drake JA (ed) Biological invasions: a global perspective. Wiley, London, pp 155–179
Maherali Z, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748
Marler MJ, Zabinski CA, Callaway RM (1999) Mycorrhizae indirectly enhance competitive effects of an invasive forb on a native bunchgrass. Ecology 1180–1186
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Morton JB (1988) Taxonomy of VA mycorrhizal fungi: classification, nomenclature, and identification. Mycotaxon 32:267–324
Mummey DL, Rillig MC (2006) The invasive plant species Centaurea maculosa alters arbuscular mycorrhizal fungal communities in the field. Plant Soil 288:81–90
Mummey DL, Stahl PD (2003) Spatial and temporal variability of bacterial 16 S rDNA-based T-RFLP patterns derived from soil of two Wyoming grassland ecosystems. FEMS Microbiol Ecol 46:113–120
O’Dea ME (2007) Influence of mycotrophy on native and introduced grass regeneration in a semiarid grassland following burning. Restor Ecol 15:149–155
Oehl F, Sieverding E, Ineichen K, Mäder P, Boller T, Wiemken A (2003) Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of central Europe. Appl Environ Microb 69:2816–2824
Opik M, Moora M, Liira J, Zobel M (2006) Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J Ecol 94:778–790
Powell JR, Parrent JL, Hart MM, Klironomos JN, Rillig MC, Maherali H (2009) Phylogenetic trait conservatism and the evolution of functional trade-offs in arbuscular mycorrhizal fungi. P Roy Soc B-Biol Sci 276:4237–4245
Pringle A, Bever JD, Gardes M, Parrent JL, Rillig MC, Klironomos JN (2009) Mycorrhizal symbioses and plant invasions. Annu Rev Ecol Evol S 40:699–715
Reinhart KO, Callaway RM (2006) Soil biota and invasive plants. New Phytol 170:445–457
Renker C, Weibhun K, Kellner H, Buscot F (2006) Rationalizing molecular analysis of field-collected roots for assessing diversity of arbuscular mycorrhizal fungi: to pool, or not to pool, that is the question. Mycorrhiza 16:525–531
Reynolds JF, Virginia RA, Kemp PR, de Soyza AG, Tremmel DC (1999) Impact of drought on desert shrubs: effects of seasonality and degree of resource island development. Ecol Monogr 69:69–106
Richardson DM, Allsopp N, D’Antonio CM, Milton SJ, Rejmanek M (2000) Plant invasions—the role of mutualisms. Biol Rev 75:65–93
Sanders IR, Fitter AH (1992) Evidence for differential responses between host–fungus combinations of vesicular-arbuscular mycorrhizas from a grassland. Mycol Res 96:415–419
Schenk NC, Smith GS (1982) Additional new and unreported species of mycorrhizal fungi (Endogonaceae) from Florida. Mycologia 77:566–574
Seifert EK, Bever JD, Maron JL (2009) Evidence for the evolution of reduced mycorrhizal dependence during plant invasion. Ecology 90:1055–1062
Shah MA, Reshi ZA, Khasa DP (2009) Arbuscular mycorrhizas: drivers or passengers of alien plant invasion. Bot Rev 75:397–417
Shah MA, Reshi ZA, Rasool N (2010) Plant invasions induce a shift in Glomalean spore diversity. Tropic Ecol 51:317–323
SimonL Lalonde M, Bruns TD (1992) Specific amplification of 18 S fungal ribosomal genes from vesicular-arbuscular mycorrhizal fungal communities. App Env Microbiol 58:291–295
Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, Thelen GC, Hallett SG, Prati D, Klironomos JN (2006) Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol 4(5):e140. doi:10.1371/journal.pbio.0040140
Stutz JC, Morton JB (1996) Successive pot cultures reveal high species richness of arbuscular endomycorrhizal fungi in arid ecosystems. Can J Bot 74:1883–1889
Sýkorová Z, Ineichen K, Wiemken A, Redecker D (2007) The cultivation bias: different communities of arbuscular mycorrhizal fungi detected in roots from the field, from bait plants transplanted to the field, and from a greenhouse trap experiment. Mycorrhiza 18:1–14
Tofts R, Silvertown J (2000) A phylogenetic approach to community assembly from a local species pool. P Roy Soc B-Biol Sci 267:363–369
Trent TD, Svejcar TJ, Blank RR (1994) Mycorrhizal colonization, hyphal lengths, and soil moisture associated with two Artemisia tridentata subspecies. Great Basin Nat 54:291–300
van der Heijden MGA, Boller T, Wiemken A, Sanders IR (1998) Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79:2082–2091
van Kleunen M, Dawson W, Schlaepfer D, Jeschke JM, Fischer M (2010) Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol Lett 13:947–958
Vogelsang KM, Bever JD (2009) Mycorrhizal densities decline in association with nonnative plants and contribute to plant invasion. Ecology 90:399–407
West NE (1983) Temperate deserts and semi-deserts, vol 5, ecosystems of the world. Elsevier, Amsterdam
Whisenant SG (1990) Changing fire frequencies on Idaho’s Snake River plains: ecological and management implications. In: McArthur ED, Romney EM, Smith SD, Tueller PT (eds) Proceedings—Symposium on Bromus Invasion, Shrub Die-off, and Other Aspects of Shrub Biology and Management. USDA Forest Service Intermountain Research Station General Technical Report INT-276, Ogden, pp 4–10
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21:213–251
Wilson GWT, Hartnett DC (1998) Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am J Bot 85:1732–1738
Young JA, Evans RA (1978) Population dynamics after wildfires in sagebrush grasslands. J Range Manage 31:283–289
Zhang Q, Yang R, Tang J, Yang H, Hu S, Chen X (2010) Positive feedback between mycorrhizal fungi and plants influences plant invasion success and resistance to invasion. PLoS One 5(8):e12380. doi:10.1371/journal.pone.0012380
Acknowledgments
The authors thank H. Varani, B. Wolk, L. Bodistow, A. Broz, and the CSU Sequencing Lab (Colorado State University); J. Morton, R. Bills, S. Purin, and B. Wheeler (INVAM); J. Bever and W. Kaonongbua (Indiana University); and N. Raizen (University of Illinois). This research was funded by the United States Army A896 Direct Funded Research Program.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 14 kb)
ESM 2
(DOCX 15 kb)
ESM 3
(DOCX 17 kb)
Fig. A1
Spatial distribution of host subsamples at each of the 3 study sites. A) CO site, B) UT site, C) WY site. Study sites are approximately 1,000 m2. Individual host subsamples do not add to 16 in most panes due to samples in close proximity to one another (< 1 m) with overlapping pins. (JPEG 88 kb)
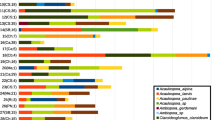
Fig. A2
Fig. A2a1 Frequency of arbuscular mycorrhizal fungi DNA sequences isolated from Bromus tectorum (a) and Artemisia tridentata (b) roots and soils across three study sites: Colorado (1), Utah (2), and Wyoming (3). Black bars indicate root associations, white bars indicate soil associations. Naming convention for the sequences are numbered based on the last 3 digits of the accession number assigned by GenBank. (JPEG 154 kb)
Rights and permissions
About this article
Cite this article
Busby, R.R., Stromberger, M.E., Rodriguez, G. et al. Arbuscular mycorrhizal fungal community differs between a coexisting native shrub and introduced annual grass. Mycorrhiza 23, 129–141 (2013). https://doi.org/10.1007/s00572-012-0455-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-012-0455-x