Abstract
Arbuscular mycorrhizal (AM) fungi and plant growth-promoting rhizobacteria (PGPR) have potential for the biocontrol of soil-borne diseases. The objectives of this study were to quantify the interactions between AM fungi [Glomus versiforme (Karsten) Berch and Glomus mosseae (Nicol. & Gerd.) Gerdemann & Trappe] and PGPR [Bacillus polymyxa (Prazmowski) Mace and Bacillus sp.] during colonization of roots and rhizosphere of tomato (Lycopersicon esculentum Mill) plants (cultivar Jinguan), and to determine their combined effects on the root-knot nematode, Meloidogyne incognita, and on tomato growth. Three greenhouse experiments were conducted. PGPR increased colonization of roots by AM fungi, and AM fungi increased numbers of PGPR in the rhizosphere. Dual inoculations of AM fungi plus PGPR provided greater control of M. incognita and greater promotion of plant growth than single inoculations, and the best combination was G. mosseae plus Bacillus sp. The results indicate that specific AM fungi and PGPR can stimulate each other and that specific combinations of AM fungi and PGPR can interact to suppress M. incognita and disease development.
Similar content being viewed by others
Introduction
That arbuscular mycorrhizal (AM) fungi contribute to the control of plant disease, and the mechanisms by which they do so have been well documented (Ahmed et al. 2009; Elsheikh and Mirghani 1997; Li and Liu 2007; Smith et al. 1986; Vierheilig et al. 2008; Whipps 2004). The presence of AM fungi in roots can reduce development of some soil-borne pathogenic bacteria, fungi, and nematodes and can also induce increased tolerance to plant diseases (Elsen et al. 2008; Liu and Chen 2007). In field experiments, Li et al. (2004) observed that the AM fungi Glomus versiforme (Karsten) Berch, Glomus mosseae (Nicol. & Gerd.) Gerdemann & Trappe, and Gigaspora rosea Nicolson & Schenck decreased the propagule density of the pathogen Fusarium oxysporum f. sp. niveum (E.F. Sm.) Snyder & Hans in the mycorrhizal roots and rhizosphere, and decreased disease incidence and severity on watermelon plants; pathogen propagule and disease reduction were greatest with G. versiforme. AM fungi have the ability to induce systemic resistance against plant-parasitic nematodes in a root system (Elsen et al. 2008). dos Anjos et al. (2010) demonstrated that the establishment of an AM fungus before nematode infection reduced reproduction of the root-knot nematode Meloidogyne incognita and reduced disease severity in infested soil. A consortium of AM fungi suppressed Fusarium wilt of cucumber and showed potential for biocontrol in greenhouse agroecosystems (Hu et al. 2010).
Like AM fungi, plant growth-promoting rhizobacteria (PGPR) can also suppress soil-borne pathogens. Becker et al. (1988) reported that the PGPR Bacillus cereus Frankland & Frankland, Bacillus sp., and two strains of Pseudomonas suppressed the plant-parasitic nematodes M. incognita, Heterodera glycines Ichinohe, Heterodera zeae Koshy, Swarup & Sethi, and Heterodera avenae Wollenweber. PGPR also suppress take-all of wheat, potato soft rot, bacterial wilt, and damping off (Weller 1988). PGPR can antagonize soil-borne pathogens in many ways (Dai et al. 2008; Preston 2004; Padgham and Sikora 2007; Tian and Robert 2000; Zhang et al. 2010).
Because both kinds of organisms depend on or are associated with plant roots, AM fungi and PGPR are likely to interact in the rhizosphere. Some PGPR produce antifungal metabolites, and it is therefore possible that PGPR could suppress AM fungi. According to Dwivedi et al. (2009), however, PGPR that produce antifungal metabolites do not necessarily interfere with the AM symbiosis and may even promote it if specific species of PGPR and AM fungus are carefully chosen for soil infestation. Evidence that the two kinds of organisms do not necessarily interfere with each other is indicated by the disease control that has been reported when AM fungi and PGPR are used together. For example, the root-rot disease complex of chickpea may be controlled by the combined use of Rhizobium, Pseudomonas striata Chester, and Glomus intraradices Schenck & Sm. (Akhtar and Siddiqui 2008). Similarly, dual inoculation with both the AM fungus G. intraradices and Pseudomonas improved soil condition and vegetable yield more than inoculation with only one of the organisms (Srivastava et al. 2007).
M. incognita is widespread and important in tomato culture, as well as in many horticultural crops in China (Fan et al. 2009; Shi et al. 2010). The current study concerns the use of PGPR and AM fungi for the control of the root-knot nematode M. incognita on vegetable crops. In addition to determining how single and combined inoculations with PGPR and AM fungi affect plant growth and disease, this study also determined how the PGPR and AM fungi affect each other.
Materials and methods
Plants, mycorrhizal inocula, PGPR, nematode inoculum, and soil
Seeds of tomato (Lycopersicon esculentum Mill) cultivar Jinguan, produced by Jinan Sun Rise Seeds Company Lt., were soaked in a 0.10% mercury bichloride solution for 10 min, washed with tap water, soaked in water at 60°C for 15 min, soaked in water at room temperature for 12 h, and germinated at 25–28°C. Germinated seeds (those with emerging radicles) were used in the experiments described in the following section. Mycorrhizal inocula were obtained from sand cultures of G. mosseae and G. versiforme (both were originally provided by the University of Western Australia, Perth, Australia) with clover (Trifolium repens L.) as the host; the inocula contained sand, spores, hyphae, and root fragments. The tested PGPR were Bacillus polymyxa (Bp) (T79) and Bacillus sp. (Bsp) (T83), both isolated from the rhizosphere of Jinguan tomato plants grown in the field and able to kill M. incognita (Mi) juveniles in previous experiments (unpublished data). Nematode eggs and second-stage juveniles (J2) were obtained from the roots of tomato cv Jinguan. Whole root systems were macerated in a 0.12 to 0.15 NaOCl solution. Nematodes were collected in a 26-μm pore sieve (500 mesh), counted, and adjusted to inoculate 3,000 nematodes per pot. The plant growth medium was a sandy loam with pH 6.7, 0.8% organic matter, 0.3 g kg−1 of total phosphorus, and 69, 43, and 38 mg kg−1 available nitrogen, phosphorus, and potassium, respectively. The soil was autoclaved at 121°C for 2 h.
Experimental design, inoculation, and plant culture
Three experiments were conducted. Experiment 1 did not include the nematode and had nine treatments: control (CK, no AM fungi or PGPR added), inoculation with G. mosseae (Gm), G. versiforme (Gv), B. polymyxa (Bp), Bacillus sp. (Bsp), Gm + Bp, Gm + Bsp, Gv + Bp, and Gv + Bsp. Plants were inoculated with AM fungi at sowing (see next paragraph) and were inoculated with PGPR immediately when seedlings at the 4-to-5 leaf stage were transplanted into pots. Experiment 2 had ten treatments: CK, inoculation with Mi, Gm + Mi, Gv + Mi, Bp + Mi, Bsp + Mi, Gm + Bp + Mi, Gm + Bsp + Mi, Gv + Bp + Mi, and Gv + Bsp + Mi. As in experiment 1, AM inocula were added when seeds were sown, while PGPR and Mi were added when seedlings at the 4-to-5 leaf stage were transplanted. Experiment 3 also had ten treatments: CK, inoculation with G. mosseae at sowing (Gm), inoculation with Bacillus sp. at transplanting (Bsp), inoculation with G. mosseae at sowing + inoculation with Bacillus sp. at transplanting (Gm + Bsp), inoculation with M. incognita at transplanting (Mi), inoculation with G. mosseae at sowing + inoculation with M. incognita at transplanting (Gm + Mi), inoculation with Bacillus sp. + M. incognita at transplanting (Bsp + Mi), inoculation with G. mosseae at sowing + inoculation with Bacillus sp. + M. incognita at transplanting (Gm + Bsp + Mi), inoculation with G. mosseae + M. incognita at transplanting (I: Gm + Mi), and inoculation with G. mosseae + Bacillus sp. + M. incognita at transplanting (II: Gm + Bsp + Mi).
Ten grams of AM fungal inocula, which contained about 500 spores, was put into a seedling-culture tray (225 cm3) and mixed with the soil; autoclaved inoculum plus 10 ml of the filter solution (free of the fungi) of the inocula were added in the control. Two germinated tomato seeds were then planted in each tray and kept in a greenhouse at 800 ± 100 μmol m−2 s−1 light intensity, light/dark 15:9 h, 28/18 ± 3°C (day/night), and 75 ± 5% relative humidity. When seedlings had four to five leaves, they were transplanted into the 3-L pots (one seedling per pot). According to the treatment, the soil in each pot was inoculated with 1.0 × 109 colony forming units (CFU) of PGPR and/or 3,000 eggs of Mi. Plants were kept in a greenhouse under the same growing conditions described above. Plants were irrigated weekly with P-free Hoagland solution or monthly with regular 1/3-strength Hoagland solution for mycorrhizal development. Plants were harvested 60 days after transplanting in experiments 1 and 2 and 0, 2, 4, 6, 9, 13, 17, 21, and 25 days after transplanting in experiment 3.
Assessment of plant growth, mycorrhizal colonization, and PGPR population
Plant height, stem diameter, number of nodes, and dry mass of shoots and roots were measured after the tomato plants were harvested. A sample of fine roots (5 g fresh mass per plant) was cut from the harvested plant, washed, cleared, and stained with acid fuchsine. AM fungal entry points, hyphae in roots, cells with arbuscules, and vesicles per unit root length were determined with a BX50 Olympus microscope equipped with a PM-30 Automatic Photomicrographic System. Mycorrhizal colonization was determined as described by Biermann and Linderman (1981). For PGPR population measurement, 1.0 g of dry soil was added with sterilized water to prepare a soil solution. PGPR numbers in the rhizosphere soil were counted with a dilution plate method 3 days after incubation under 30°C (Bashan et al. 1993; Zhao and He 2002).
Assessment of root-knot nematode damage
Another sample of fine roots (5 g fresh mass per plant) was boiled in 0.1% acid fuchsine–lactophenol for 3 min and cleared in lactophenol for 2 days (Byrd et al. 1983). The numbers of nematodes in the roots (juveniles and females/gram root) were then determined by examining the roots with a light microscope (Liu 1995). Penetration rate was calculated as the number of M. incognita juveniles and females in the root system/3,000 (the number of eggs added per plant) × 100 (Caroli et al. 1996). After the egg masses were counted, up to ten egg masses per plant (egg masses were not always present depending on the treatment and harvest date) were soaked in a 5% sodium hypochlorite solution for 2–5 min, and the number of eggs per egg mass was determined.
Nematode galling was assessed according to a 0–6 root galling index: 0 = no galls formed; 0.5 = less than 10% of the roots with galls, galls are small, fine roots appear normal; 1 = less than 10% of the roots with galls; 2 = 10% to 20% of the roots with galls; 3 = 20% to 50% of the roots with galls and severe root swelling affecting less than 30% of the root system; 4 = 50% to 70% of the roots with galls and severe root swelling affecting 30% to 70% of the root system; 5 = 70% to 90% of the roots with galls, severe root swelling affecting greater than 70% of the root system, and fine roots are rare; 6 = 100% of the roots with galls, severe swelling affecting the entire root system, fine roots are absent, and root rot is evident. Disease indexes (percent) = ∑ (numbers of plants in each grade × the representative value of each grade/(total numbers of plants × the representative value of the highest grade) × 100; incidence (percent) = numbers of diseased plants/total plants and relative control (percent) = 1 − (disease index of treatment/disease index with Mi alone) × 100, as described by Fang (1998).
Statistical analysis
Treatments were replicated five times in experiments 1 and 2. In experiment 3, each combination of treatment and nine harvest times was replicated three times. Any data in percent were transformed before statistical analysis. Data were analyzed by ANOVA. Differences in means were compared with Duncan's new multiple range test and considered significant at P ≤ 0.05. Costat (CoHort Software, Berkeley, CA, USA) and 2003 Microsoft® Excel were used for statistical analyses.
Results
Experiment 1: colonization of roots and rhizosphere by AM fungi and PGPR, and plant growth
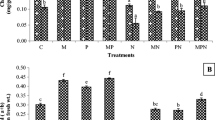
Colonization of roots by AM fungi was greater with G. mosseae + B. polymyxa and G. mosseae + Bacillus sp. than with G. mosseae or G. versiforme alone, and colonization was greatest with G. mosseae + Bacillus sp. (Fig. 1). There was no mycorrhizal colonization in the noninoculated plants.
Mycorrhizal percentage of tomato (L. esculentum Mill) seedlings inoculated with arbuscular mycorrhizal fungi in experiment 1. Gv G. versiforme (Karsten) Berch, Gm G. mosseae (Nicol. & Gerd.) Gerdemann & Trappe, Bp B. polymyxa (Prazmowski) Mace (T79), Bsp Bacillus sp.(T83). Tomato seeds were planted in soil inoculated with the arbuscular mycorrhizal fungi (500 spores), and the seedlings were inoculated with the bacteria (1.0 × 109 CFU/ml, 10 ml per plant) immediately when they were transplanted into pots. Values are means of five replicates
The population density of B. polymyxa or Bacillus sp. in the rhizosphere was two to five times greater in the treatment with the bacteria added with G. mosseae or G. versiforme than in the treatment with the bacteria alone (Fig. 2). No PGPR were detected in the rhizosphere of the noninoculated plants.
Population density of PGPR in the rhizosphere of tomato seedlings in experiment 1. Gv G. versiforme (Karsten) Berch, Gm G. mosseae (Nicol. & Gerd.) Gerdemann & Trappe, Bp B. polymyxa (Prazmowski) Mace(T79), Bsp Bacillus sp.(T83). Tomato seeds were planted in soil without or with the arbuscular mycorrhizal fungi (500 spores) and were inoculated with the bacteria (1.0 × 109 CFU/ml, 10 ml per plant) as indicated when the seedlings were transplanted into pots. Values are means of five replicates
Plant height, stem diameter, number of nodes, and dry weight of the tops and roots were significantly higher with G. mosseae + Bacillus sp. than with the noninoculated plants or with other single inoculations. The dry weight of the tops in single inoculations was greater than that of the noninoculated plants (Table 1).
Experiment 2: effects of AM fungi and PGPR on the root-knot nematode and galling of roots
Plant growth was lower in the treatment with M. incognita than in the noninoculated plants but was greater in the treatment with M. incognita added with AM fungi, PGPR, or AM fungi + PGPR than in the treatment with M. incognita (Table 2), while disease incidences and disease indexes were all significantly lower in the treatment with M. incognita added with AM fungi, PGPR, and AM fungi + PGPR than in the treatment with M. incognita (Table 3). Relative control was greatest in Gm + Bsp, Gv + Bsp, and Gm + Bp treatments (Table 3). The numbers of J2, females, total nematodes, egg masses, and eggs per egg mass were lower in the treatment with M. incognita added with AM fungi or PGPR than in the treatment with M. incognita (Table 4). The numbers of J2 and eggs per egg mass were lower in Gm + Bsp + Mi treatment than in any other treatment (Table 4).
Experiment 3: M. incognita colonization as affected by AM fungi and PGPR
When M. incognita was added alone, the number of M. incognita in roots increased rapidly between days 0 and 2 and slowly thereafter (Fig. 3). By day 25, the number of nematodes in roots was in the following order by treatment (from highest to lowest): Mi, I Gm + Mi, Bsp + Mi, II Gm + Bsp + Mi, Gm + Mi, and Gm + Bsp + Mi (Fig. 3). In other words, penetration of roots by M. incognita was lowest with the combination of G. mosseae added at sowing and Bacillus sp. and M. incognita added at transplanting.
Penetration rate of M. incognita on tomato roots as affected by time after inoculation with arbuscular mycorrhizal fungi and PGPR in experiment 3. Mi plants inoculated with 3,000 eggs of M. incognita at transplanting. Bsp + Mi plants inoculated with Bacillus sp. (1.0 × 109 CFU/ml × 10 ml per plant) and M. incognita at transplanting. Gm + Mi seeds sowed in soil containing 500 spores of G. mosseae + M. incognita at transplanting. Gm + Bsp + Mi seeds sowed in soil containing G. mosseae + Bacillus sp. + M. incognita at transplanting. I Gm + Mi plants inoculated with G. mosseae + M. incognita at transplanting. II Gm + Bsp + Mi plants inoculated with G. mosseae + Bacillus sp. + M. incognita at transplanting. Penetration rate was calculated as the number of nematodes in a root system/3,000 (the number of eggs added per root system) × 100. Values are means of three replicates
Discussion
Multimicrobial inoculation has been proposed as a way of protecting plants against environmental stress and increasing the sustainability of plant production. AM fungi and PGPR are well recognized as microorganisms that can improve plant nutrition and growth and also reduce plant disease, and they are usually more effective when added together than alone (Miroslav and Milan 2000; Attia and Awad 2003; Akhtar and Siddiqui 2008; Siddiqui and Akhtar 2009). For instance, dual inoculation with the AM fungus Glomus fasciculatum and the bacterium Azospirillum brasilense enhanced nitrogen acquisition and growth of Medicago sativa L. (Biró et al. 2000). In the present study, tomato plant growth was improved more by dual inoculation with AM fungi and PGPR than by single inoculations, and enhancement was greater with the dual inoculation of G. mosseae and Bacillus sp. than with other dual or single inoculations. Single inoculations were consistently effective at increasing shoot growth but did not improve root size. Meyer and Linderman (1986) found that plant growth and nodulation of subterranean clover were enhanced by indigenous AM fungi and Pseudomonas putida, and the PGPR increased colonization by AM fungi, while the latter did not increase populations of the former. The results of the present study not only indicate that the tested PGPR, B. polymyxa and Bacillus sp., increased AM fungal colonization of roots, but also that the AM fungi increased population of PGPR in the rhizosphere. So the AM fungi and PGPR can stimulate each other and play a synergistic activity and function in improving plant growth and in reducing plant disease.
Although the effects of single inoculations with AM fungi or PGPR on pathogens and disease have often been studied (Barea et al. 2002; Cooper and Grandisons 1986; Padgham and Sikora 2007; Preston 2004; Shreenivasa et al. 2007a; Wang and Hu 2000), the effects of dual inoculations on plant pathogens and plant diseases have seldom been studied. In one study, dual inoculation with Bacillus subtilis Ehrenberg M3 and G. mosseae BEG29 decreased crown rot shoot symptoms as well as the numbers of Phytophthora cactorum (Lebert & Cohn) Schröt oospores in the roots of strawberry in autumn, while in the summer, root necrosis was slightly decreased only by B. subtilis and G. mosseae + Gliocladium catenulatum Gilman & Abbott (Vestberg et al. 2004). Serfoji et al. (2010) observed that application of vermicompost + Glomus aggregatum + Bacillus coagulans increased plant growth characters and reduced root-knot index, nematode reproduction rate, number of galls, and egg masses on tomato cv Pusa Ruby in sandy loam acidic soils. In the present experiments, nematode penetration in roots was similar for most of the treatments as recorded in the second day (Fig. 3). However, root colonization by AM fungi and PGPR, individually or in combination, had a negative effect on the development of M. incognita following the second day of inoculation and successive harvest dates. Nematode population build up decreased with time as indicated by the differences in nematode development in the roots for the different treatments. So we concluded that nematode penetration of roots, nematode reproduction, and nematode-incited disease were decreased more by dual inoculations with AM fungi and PGPR than by single inoculations, and dual inoculation with G. mosseae and Bacillus sp. gave the best results. This may be due to AM fungus's strong induction of systemic resistance in plants towards nematodes (Elsen et al. 2008), and PGPR can do this through their own metabolism (such as phosphate solubilization, hormone production, N2-fixation, etc.), directly affect the plant metabolism (for instance, enhance water and mineral uptake, improve root development, enhance plant enzyme activity, etc.), or affect the plant by "helping" another beneficial microorganism to function better (e.g., Azospirillum increasing modulation of legumes by rhizobia, or enhancing mycorrhizal phosphate solubilization or mycorrhizal colonization) (Bashan and Holguin 1998; Gryndler 2000; Linderman 1988; Shreenivasa et al. 2007b; Fig. 1, Tables 1 and 2). However, the mechanisms of nematode suppression in the roots are unknown but would seem to be related to physiological changes in roots affecting nematode food source or feeding (unfavorable condition for nematode development) rather than a direct competition for space. de la Peña et al. (2006) even considered that nematode suppression by AM fungi did not occur through a systemic plant response but through local mechanisms. If the experiments were carried out in non-sterile soil simulating field conditions, what would be the results? Sometimes the addition of PGPR does not seem to improve the results of AM fungi single treatments in terms of nematode infection (Jaizme-Vega et al. 2006). So the mechanisms by which AM fungi and PGPR interact to decrease plant disease also require additional study.
It is important to evaluate and select the best combination of microorganisms to use in dual or tri-inoculations with AM fungi and PGPR. The “best combination” must be established on the basis of inoculation time and method, host plant, pathogen, and ecological conditions. Although the best combination was indicated for experimental conditions and organisms in the current study, further investigation is needed on a wider range of AM fungi (Tchabi et al. 2010), PGPR, plant species (Powell et al. 2009), and pathogens. Even though there were experiments which showed that many AM fungi and PGPR successfully improved growth and reduced root knot nematode infestation and they could be applied as a biofertilizer, as well as a biocontrol agent against the root-knot nematode (Ahmed et al. 2009; Elsheikh and Mirghani 1997), there will still be a long way to go before AMF + PGPB becomes a promoting technique for sustainable agricultural production.
References
Ahmed SH, Abdelgani ME, Yassin AM (2009) Effects of interaction between vesicular-arbuscular mycorrhizal (VAM) fungi and root-knot nematodes on Dolichos bean (Lablab niger Medik.) plants. AEJSA 3:678–683
Akhtar MS, Siddiqui ZA (2008) Biocontrol of a root-rot disease complex of chickpea by Glomus intraradices, Rhizobium sp. and Pseudomonas striata. Crop Protect 27:410–417
Attia M, Awad NM (2003) Assessment the impact of certain growth promoting rhizobacteria strains on symbiotic effectiveness of arbuscular mycorrhizal fungi. Egypt J Microbiol 38:75–88
Barea JM, Toro M, Orozco MO, Campos E, Azcón R (2002) The application of isotopic 32P and 15N-dilution techniques to evaluate the interactive effect of phosphate-solubilizing rhizobacteria, mycorrhizal fungi and Rhizobium to improve the agronomic efficiency of rock phosphate for legume crops. Nutr Cycl Agroecosyst 63:35–42
Bashan Y, Holguin G (1998) Proposal for the division of plant growth-promoting rhizobacteria into classifications: Biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol Biochem 30:1225–1228
Bashan Y, Holguin G, Lifshitz R (1993) Isolation and characterization of plant growth-promoting rhizobacteria. In: Glick BR, Thompson JE (eds) Methods in plant molecular biology and biotechnology. CRC Press, Boca Raton, pp 331–345
Becker JO, Zavaleta-Mejia E, Colbert SF, Schroth MN, Weinhold AR, Hancock JG, Van Gundy SD (1988) Effects of rhizobacteria on root-knot nematodes and gall formation. Phytopathology 78:1466–1469
Biermann B, Linderman RG (1981) Quantifying vercular-arbuscular mycorrhizas: a proposed method towards standardization. New Phytol 87:63–67
Biró B, Koves-Pechy K, Vörös I, Takács T, Eggenberger P, Strasser RJ (2000) Interrelations between azospirillum and rhizobium nitrogen-fixers and arbuscular mycorrhizal fungi in the rhizosphere of alfalfa in sterile, AMF-free or normal soil conditions. Appl Soil Ecol 15:159–168
Byrd DW, Kirkpatrick JT, Barker KR (1983) An improved technique for clearing and staining plant tissues for detection of nematodes. J Nematol 15:142–143
Caroli L, Glazer I, Gaugler R (1996) Entomopathogenic nematode infectivity assay: comparison of penetration rate into different hosts. Biocontrol Sci Technol 6:227–233
Cooper KM, Grandisons GS (1986) Interaction of vesicular-arbuscular mycorrhizal fungi and root-knot nematode on cultivars of tomato and white clover susceptible to Meloidogyne hapla. Annu Appl Biol 108:555–565
Dai M, Wang HX, Yin YY, Wu X, Liu RJ (2008) Effects and mechanisms of interactions between arbuscular mycorrrhizal fungi and plant growth promoting rhizobacteria. Acta Ecologica Sinica 28:2854–2860 (In Chinese with English abstract)
de la Peña E, Echeverría SR, van der Putten WH, Moens FHM (2006) Mechanism of control of root-feeding nematodes by mycorrhizal fungi in the dune grass Ammophila arenaria. New Phytol 169:829–840
dos Anjos ECT, Cavalcante UMT, Gonçalves DMC, Pedrosa EMR, dos Santos VF, Maia LC (2010) Interactions between an arbuscular mycorrhizal fungus (Scutellospora heterogama) and the root-knot nematode (Meloidogyne incognita) on sweet passion fruit (Passiflora alata). Braz Arch Biol Technol 53:801–809
Dwivedi D, Johri BN, Ineichen K, Wray V, Wiemken A (2009) Impact of antifungals producing rhizobacteria on the performance of Vigna radiata in the presence of arbuscular mycorrhizal fungi. Mycorrhiza 19:559–570
Elsen A, Gervacio D, Swennen R, Waele DD (2008) AMF-induced biocontrol against plant parasitic nematodes in Musa sp.: a systemic effect. Mycorrhiza 18:251–256
Elsheikh EAE, Mirghani AMO (1997) Interaction of VA mycorrhiza and root-knot nematode on tomato plants—effects of nematode inoculum density, soil texture and soil sterilization. Jonares 1:1–6
Fan YL, Zhang WG, Lu SH, Gao XY, Liu LK (2009) Identification of the root-knot nematode from vegetables in greenhouses in Shandong. Acta Agriculturae Boreali-Sinica 24(Suppl):262–264 (In Chinese with English abstract)
Fang ZD (1998) Research methods of plant pathology (in Chinese), 3rd edn. China Agri Press, Beijing, pp p11–p12
Gryndler M (2000) Interactions of arbuscular mycorrhizal fungi with other soil organisms. In: Kapulnik Y, Douds DD Jr (eds) Arbuscular mycorrhizas: physiology and function. Kluwer, Dordrecht, pp 239–262
Hu JL, Lin XG, Wang JH, Shen WH, Wu S, Peng SP, Mao TT (2010) Arbuscular mycorrhizal fungal inoculation enhances suppression of cucumber Fusarium wilt in greenhouse soils. Pedosphere 20:586–593
Jaizme-Vega MC, Rodriguez-Romero AS, Nunez LAB (2006) Effect of the combined inoculation of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria on papaya (Caria papaya L.) infected with root-knot nematode Meloidogyne incognita. Fruits 61:151–162
Li JX, Liu RJ (2007) Potential of mycorrhizal fungal agents on controlling soil-borne plant diseases. Acta Phytopathologica Sinica 37:1–8 (In Chinese with English abstract)
Li M, Liu RJ, Li XL (2004) Influences of arbuscular mycorrhizal fungi on growth and Fusarium-wilt disease of watermelon in field. Acta Phytopathologica Sinica 34:472–473 (in Chinese with English abstract)
Linderman RG (1988) Mycorrhizal intercations with the rhizopshere microflora: the mycorrhizosphere effect. Phytopathology 78:366–371
Liu WZ (1995) Research techniques of plant nematology (in Chinese). Liaoning Science Technol Press, Shenyang, pp 1–242
Liu RJ, Chen YL (2007) Mycorrhizaology (in Chinese). Science Press, Beijing, pp 208–209
Meyer JR, Linderman RG (1986) Response of subterranean clover to dual inoculation with vesicular- arbuscular mycorrhizal fungi and a plant growth-promoting bacterium Pseudomonas putida. Soil Biol Biochem 18:185–190
Miroslav V, Milan G (2000) Response of micropropagated potatoes transplanted to peat media to post-vitro inoculation with arbuscular mycorrhizal fungi and soil bacteria. Appl Soil Ecol 15:145–152
Padgham JL, Sikora RA (2007) Biological control potential and modes of action of Bacillus megaterium against Meloidogyne graminicola on rice. Crop Prot 26:971–977
Powell JR, Campbell RG, Dunfield KE, Gulden RH, Hart MM, Levy-Booth DJ, Klironomos JN, Pauls KP, Swanton CJ, Trevors JT, Antunes PM (2009) Effect of glyphosate on the tripartite symbiosis formed by Glomus intraradices, Bradyrhizobium japonicum, and genetically modified soybean. Appl Soil Ecol 41:128–136
Preston GM (2004) Plant perceptions of plant growth-promoting Pseudomonas. Philos Trans R Soc Lond B Biol Sci 359:907–918
Serfoji P, Rajeshkumar S, Selvaraj T (2010) Management of root-knot nematode, Meloidogyne incognita on tomato cv Pusa Ruby. by using vermicompost, AM fungus, Glomus aggregatum and mycorrhiza helper bacterium, Bacillus coagulans. J Agric Technol 6:37–45
Shi LB, Wang ZH, Wu HY, Liu J (2010) Influence of continuous tomato-cropping on second-stage juveniles of root-knot nematode and free-living nematodes from rhizosphere soil in plastic greenhouse. Acta Phytopathologica Sinica 40:81–89 (In Chinese with English abstract)
Shreenivasa KR, Krishnappa K, Ravichandra NG (2007a) Interaction effects of arbuscular mycorrhizal fungus Glomus fasciculatum and root–knot nematode, Meloidogyne incognita on growth and phosphorous uptake of tomato. Karnataka J Agric Sci 20:57–61
Shreenivasa KR, Krishnappa K, Ravichandra NG (2007b) Survival and penetration of Meloidogyne incognita larvae in tomato roots in presence of arbuscular mycorrhizal fungus, Glomus fasciculatum. Karnataka J Agric Sci 20:166–16
Siddiqui ZA, Akhtar MS (2009) Effects of antagonistic fungi, plant growth-promoting rhizobacteria, and arbuscular mycorrhizal fungi alone and in combination on the reproduction of Meloidogyne incognita and growth of tomato. J Gen Plant Pathol 75:144–153
Smith GS, Rongadori RW, Hussey RS (1986) Interaction of endomycorrhizal fungi, superphosphate, and Meloidogyne incognita on cotton in microplot and field studies. J Nematol 18:208–216
Srivastava R, Roseti D, Sharma AK (2007) The evaluation of microbial diversity in a vegetable based cropping system under organic farming practices. Appl Soil Ecol 36:116–123
Tchabi A, Coyne D, Hountondji F, Lawouin L, Wiemken A, Oehl F (2010) Efficacy of indigenous arbuscular mycorrhizal fungi for promoting white yam (Dioscorea rotundata) growth in West Africa. Appl Soil Ecol 45:92–100
Tian H, Robert DR (2000) Effects of rhizobacteria on soybean cyst nematode, Heterodera glycines. J Nematol 32:377–388
Vestberg A, Kukkonen S, Saari K, Parikka P, Huttunen J, Tainio L, Devos D, Weekers F, Kevers C, Thonart P, Lemoine MC, Cordier C, Alabouvette C, Gianinazzi S (2004) Microbial inoculation for improving the growth and health of micropropagated strawberry. Appl Soil Ecol 27:243–258
Vierheilig H, Steinkellner S, Khaosaad T, Garcia-Garrido JM (2008) The biocontrol effect of mycorrhization on soil-borne fungal pathogens and the autoregulation of the AM symbiosis: one mechanism, two effects? In: Varma A (ed) Mycorrhiza: genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics. Springer, Heidelberg, pp 307–320
Wang YL, Hu ZJ (2000) Effect of VA mycorrhiza on nematodiasis of tomato. J Huazhong Agric Uni 19:25–28 (In Chinese with English abstract)
Weller DM (1988) Biological control of soil-borne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol 26:397–407
Whipps JM (2004) Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can J Bot 82:1198–1227
Zhang S, White TL, Martinez MC, McInroy JA, Kloepper JW, Klassen W (2010) Evaluation of plant growth-promoting rhizobacteria for control of Phytophthora blight on squash under greenhouse conditions. Biol Control 53:129–135
Zhao B, He SJ (2002) Microbiology experiments (in Chinese). Science Press, Beijing, pp 23–54
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (30871737) and the Sub-project in Industry Program of Ministry of Agriculture (nyyzx 07-050-5).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, R., Dai, M., Wu, X. et al. Suppression of the root-knot nematode [Meloidogyne incognita (Kofoid & White) Chitwood] on tomato by dual inoculation with arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria. Mycorrhiza 22, 289–296 (2012). https://doi.org/10.1007/s00572-011-0397-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-011-0397-8