Abstract
Background
Real-world data on the efficacy and safety of sofosbuvir plus velpatasvir (SOF/VEL) treatment for patients with hepatitis C virus (HCV)-related decompensated cirrhosis are limited in Japan.
Methods
A total of 190 patients with compensated (108) or decompensated (82) cirrhosis who initiated direct-acting antiviral (DAA) treatment between February 2019 and August 2019 were enrolled. Sustained virologic response (SVR) was defined as undetectable serum HCV-RNA at 12 weeks after the end of treatment (EOT).
Results
The SVR12 rates were 92.6% in patients with compensated cirrhosis and 90.2% in patients with decompensated cirrhosis (p = 0.564), and the treatment completion rates were 98.1% and 96.3%, respectively (p = 0.372). In patients with decompensated cirrhosis, 3 patients discontinued treatment and 2 patients died because of liver-related events. In patients with decompensated cirrhosis with SVR12, 50% of patients with Child–Pugh class B at baseline showed improvement to class A at SVR12, and 27% and 9% of patients with Child–Pugh class C at baseline showed improvement to class B and class A at SVR12, respectively. Patients who achieved SVR12 showed elevated serum albumin levels at the EOT, which were further elevated at SVR12, but no elevated serum albumin levels after the EOT were observed in patients with baseline serum albumin levels less than 2.8 g/dl.
Conclusions
Real-world efficacy of SOF/VEL treatment for patients with decompensated cirrhosis was similar to Japanese phase 3 study, although treatment discontinuation and death related to liver disease occurred. In patients with poor hepatic reserve, whether it improves continuously after viral clearance requires further evaluation.



Similar content being viewed by others
Abbreviations
- HCV:
-
Hepatitis C virus
- DAA:
-
Direct-acting antiviral
- SOF:
-
Sofosbuvir
- NS:
-
Nonstructural
- VEL:
-
Velpatasvir
- RBV:
-
Ribavirin
- SVR:
-
Sustained virologic response
- LDV:
-
Ledipasvir
- EBR:
-
Elbasvir
- GZR:
-
Grazoprevir
- GLE:
-
Glecaprevir
- PIB:
-
Pibrentasvir
- EOT:
-
End of treatment
- RM ANOVA:
-
Repeated measures analysis of variance
- MELD:
-
Model for end-stage liver disease
- IQR:
-
Interquartile range
References
Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–10.
Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–46.
Planas R, Balleste B, Alvarez MA, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol. 2004;40:823–30.
Tsuji K, Kurosaki M, Itakura J, et al. Real-world efficacy and safety of ledipasvir and sofosbuvir in patients with hepatitis C virus genotype 1 infection: a nationwide multicenter study by the Japanese Red Cross Liver Study Group. J Gastroenterol. 2018;53:1142–50.
Tahata Y, Sakamori R, Urabe A, et al. Liver fibrosis is associated with corrected QT prolongation during ledipasvir/sofosbuvir treatment for patients with chronic hepatitis C. Hepatol Commun. 2018;2:884–92.
Ogawa E, Furusyo N, Nakamuta M, et al. Glecaprevir and pibrentasvir for Japanese patients with chronic hepatitis C genotype 1 or 2 infection: results from a multicenter, real-world cohort study. Hepatol Res. 2019;49:617–26.
Toyoda H, Atsukawa M, Takaguchi K, et al. Real-world virological efficacy and safety of elbasvir and grazoprevir in patients with chronic hepatitis C virus genotype 1 infection in Japan. J Gastroenterol. 2018;53:1276–84.
Mashiba T, Joko K, Kurosaki M, et al. Real-world efficacy of elbasvir and grazoprevir for hepatitis C virus (genotype 1): a nationwide, multicenter study by the Japanese Red Cross Hospital Liver Study Group. Hepatol Res. 2019;49:1114–20.
Tamori A, Inoue K, Kagawa T, et al. Intention-to-treat assessment of glecaprevir + pibrentasvir combination therapy for patients with chronic hepatitis C in the real world. Hepatol Res. 2019;49:1365–73.
Curry MP, O'Leary JG, Bzowej N, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373:2618–28.
Panel A-IHG. Hepatitis C Guidance. Update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis. 2018;2018(67):1477–92.
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL recommendations on treatment of hepatitis C. J Hepatol. 2018;69:461–511.
Takehara T, Sakamoto N, Nishiguchi S, et al. Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: an open-label phase 3 trial. J Gastroenterol. 2019;54:87–95.
Tanaka A, Drafting Committee for Hepatitis Management Guidelines tJSoH. JSH Guidelines for the Management of Hepatitis C Virus Infection: 2019 Update. Hepatol Res 2020.
Seko Y, Moriguchi M, Hara T, et al. Presence of varices in patients after hepatitis C virus eradication predicts deterioration in the FIB-4 index. Hepatol Res. 2019;49:473–8.
Tsuji S, Uchida Y, Uemura H, et al. Involvement of portosystemic shunts in impaired improvement of liver function after direct-acting antiviral therapies in cirrhotic patients with hepatitis C virus. Hepatol Res. 2020;50:512–23.
Foster GR, Irving WL, Cheung MC, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224–311.
Belli LS, Berenguer M, Cortesi PA, et al. Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: a European study. J Hepatol. 2016;65:524–31.
Cheung MCM, Walker AJ, Hudson BE, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741–7.
Gentile I, Scotto R, Coppola C, et al. Treatment with direct-acting antivirals improves the clinical outcome in patients with HCV-related decompensated cirrhosis: results from an Italian real-life cohort (Liver Network Activity-LINA cohort). Hepatol Int. 2019;13:66–74.
El-Sherif O, Jiang ZG, Tapper EB, et al. Baseline factors associated with improvements in decompensated cirrhosis after direct-acting antiviral therapy for hepatitis C virus infection. Gastroenterology. 2018;154(2111–2121):e2118.
Fernandez Carrillo C, Lens S, Llop E, et al. Treatment of hepatitis C virus infection in patients with cirrhosis and predictive value of model for end-stage liver disease: analysis of data from the Hepa-C registry. Hepatology. 2017;65:1810–22.
Asahina Y, Drafting Committee for Hepatitis Management Guidelines tJSoH. JSH Guidelines for the Management of Hepatitis C Virus Infection, 2019 Update; Protective Effect of Antiviral Therapy against Hepatocarcinogenesis. Hepatol Res 2020.
Acknowledgements
The authors thank Yoshito Uchida (Department of Gastroenterology and Hepatology, Saitama Medical University), Sawako Uchida (Department of Hepatology, Graduate School of Medicine, Osaka City University), Shinnya Maekawa (First Department of Internal Medicine, Faculty of Medicine, University of Yamanashi), Kotaro Kumagai (Digestive and Lifestyle Diseases, Department of Human and Environmental Sciences, Kagoshima University Graduate School of Medicine and Dental Sciences), Hiroaki Takatani (Department of Gastroenterology, Nara Medical University), Yohei Koizumi (Department of Gastroenterology and Metabology, Ehime University Graduate School of Medicine), Hidekatsu Kuroda (Division of Hepatology, Deportment of Internal Medicine, Iwate Medical University), Shun Kaneko (Department of Gastroenterology and Hepatology, Musashino Red Cross Hospital), Satoru Hashimoto, Kazumasa Tajima (Clinical Research Center, National Hospital Organization Nagasaki Medical Center), Tatsuya Minami (Department of Gastroenterology, Graduate School of Medicine, The University of Tokyo), Kazuo Okumoto (Department of Gastroenterology, Faculty of Medicine, Yamagata University), Yuya Seko (Department of Molecular Gastroenterology and Hepatology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine), Yosuke Osawa (The Research Center for Hepatitis and Immunology, National Center for Global Health and Medicine), Tatsushi Naito (Second Department of Internal Medicine, Faculty of Medical Sciences, University of Fukui), Masato Nakamura (Department of Gastroenterology, Graduate School of Medicine, Chiba University), Miyako Murakawa (Department of Gastroenterology and Hepatology, Department of Liver Disease Control, Tokyo Medical and Dental University,), Atsunori Tsuchiya (Division of Gastroenterology and Hepatology, Graduate School of Medicine and Dental Sciences, Niigata University), Masanori Fukushima (Department of Gastroenterology and Hepatology, Nagasaki University of Graduate School of Biomedical Sciences), Tatsunori Hanai (Department of Gastroenterology/Internal Medicine Gifu University Graduate School of Medicine) and Takuro Hisanaga (Department of Gastroenterology and Hepatology, Yamaguchi University Graduate School of Medicine) for data collection and Rina Okada (Department of Gastroenterology and Hepatology, Osaka University Graduate School of Medicine) and Hiroyuki Araki (Osaka University Graduate School of Medicine Department of Biostatistics and Data Science) for data management.
Funding
This work was partially supported by a Grant-in-Aid for Research on Hepatitis from the Ministry of Health Labor and Welfare of Japan, and the Japan Agency for Medical Research and Development (JP20fk0210058), and Gilead Sciences, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Satoshi Mochida received grants from Kowa Co, Ltd. CMIC Co., Ltd, AbbVie GK, Janssen Pharmaceutical K. K., EA Pharma Co, Ltd MIC Medical Corp. Sumitomo Dainippon Pharma Co., Ltd, Mochida Pharmaceutical Co., Ltd. Daiichi Sankyo Co., Ltd, Toray Indutries Inc, Chugai Pharmaceutical Co., Ltd, Asuka Pharmaceutical Co., Ltd, Eisai Co., Ltd. and Gilead Sciences, Inc. and is on the speakers’ bureau for Gilead Sciences, Inc., Bristol-Myers Squibb Company, MSD, Otuka Pharmaceutical Co., Ltd. Sumitomo Dainippon Pharma Co., Ltd. Asuka Pharmaceutical Co., Ltd and AbbVie GK and received patent royalties from SRL Inc. Norifumi Kawada received grants from Gilead Sciences, Inc. and AbbVie GK. and is on the speakers’ bureau for Gilead Sciences, Inc., MSD and AbbVie GK.. Nobuyuki Enomoto received grants from Gilead Sciences, Inc. and is on the speakers’ bureau for Gilead Sciences, Inc.. Akio Ido received grants from AbbVie GK. and is on the speakers’ bureau for Gilead Sciences, Inc., Bristol-Myers Squibb Company and AbbVie GK.. Masayuki Kurosaki is on the speakers’ bureau for Gilead Sciences, Inc. Hiroshi Yatsuhashi received grants from AbbVie GK. Yoshiyuki Ueno received grants from EA Pharma Co and AbbVie GK. and is on the speakers’ bureau for AbbVie GK., EA Pharma Co, and Otsuka Pharmaceutical Co. Yoshito Itoh received grants from AbbVie GK, Bristol-Myers Squibb Company, Gilead Sciences, Inc and MSD and is on the speakers’ bureau for Gilead Sciences, Inc., Bristol-Myers Squibb Company, MSD and AbbVie GK.. Tatsuya Kanto is on the speakers’ bureau for Gilead Sciences, Inc.and MSD. Goki Suda received grants from Gilead Sciences, Inc.. Naoya Kato received grants from Gilead Sciences, Inc., Bristol-Myers Squibb Company and AbbVie GK. and is on the speakers’ bureau for Gilead Sciences, Inc., MSD, Bristol-Myers Squibb Company and AbbVie GK.. Yasuhiro Asahina belongs to a donation-funded department funded by Toray Industries Inc, Gilead Sciences Inc, AbbVie GK, and Fujirebio Inc, Chugai Pharmaceutical Co. Ltd. and MSD. Shuji Terai received grants from Intersterm Routo, Asuka, Tsumura, Chiome Biosciense, Systemx, Touso, Abbvie, Takeda, Eisai, Kyowa, BioMimetics Sympathies and Daiichisannkyo and is on the speakers’ bureau for Gilead Sciences, Inc., Asuka, MSD, Otsuka, Daiichisannkyo and Takeda. Norio Akuta is on the speakers’ bureau for Gilead Sciences, Inc., Bristol-Myers Squibb Company, MSD, Dainippon Sumitomo Pharma, Mitsubishi Tanabe Pharma Co. Ltd and AbbVie GK. Takahiro Kodama received grants from Gilead Sciences, Inc. and AbbVie GK.. Tetsuo Takehara received grants from Gilead Sciences, Inc., MSD, and AbbVie GK. and is on the speakers’ bureau for Gilead Sciences, Inc., MSD, and AbbVie GK.. All other authors declare that they have no conflicts of interest to disclose.
The trial registration number
University Hospital Medical Information Network (UMIN: 000036150).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2020_1733_MOESM1_ESM.tif
Supplementary file1 Figure 1. Treatment completion rates according to liver cirrhosis. Gray box, patients with compensated cirrhosis. White box, patients with decompensated cirrhosis. (TIF 852 kb)
535_2020_1733_MOESM2_ESM.tif
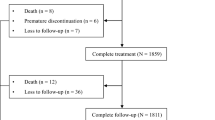
Supplementary file2 Figure 2. Flow chart of patients who were included in the examination of the changes in liver function during follow-up. (TIF 1142 kb)
535_2020_1733_MOESM3_ESM.tif
Supplementary file3 Figure 3. Changes in liver function score at SVR12 from baseline among patients with SVR12 according to liver cirrhosis. A Child-Pugh score. bMELD score. Gray box, patients with compensated cirrhosis. White box, patients with decompensated cirrhosis. (TIF 864 kb)
535_2020_1733_MOESM4_ESM.tif
Supplementary file4 Figure 4. Changes in Child-Pugh score of each liver function according to liver cirrhosis and baseline Child-Pugh score among patients with SVR12. A Albumin level. B Total bilirubin level. C Prothrombin activation. D Ascites. E Encephalopathy (TIF 1356 kb)
Rights and permissions
About this article
Cite this article
Tahata, Y., Hikita, H., Mochida, S. et al. Sofosbuvir plus velpatasvir treatment for hepatitis C virus in patients with decompensated cirrhosis: a Japanese real-world multicenter study. J Gastroenterol 56, 67–77 (2021). https://doi.org/10.1007/s00535-020-01733-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-020-01733-4