Abstract
Background
This study examined the effects of peretinoin, an acyclic retinoid, on the survival of patients with hepatitis C virus-related hepatocellular carcinoma (HCC) who had completed curative therapy and participated in a randomized, placebo-controlled trial.
Methods
This study was an investigator-initiated retrospective cohort study. Subjects were all patients who were administered the investigational drug (peretinoin 600 mg/day, peretinoin 300 mg/day, or placebo) in the randomized trial. Survivals between the groups were compared using the log-rank test, and hazard ratios were estimated by Cox regression.
Results
Survey data were collected from all patients (n = 392) who participated in the randomized trial, all of whom were then divided into the peretinoin 600 mg/day (n = 132), peretinoin 300 mg/day (n = 131), and placebo (n = 129) groups. At the median follow-up of 4.9 years, 5-year cumulative survival rates for patients in the 600 mg/day, 300 mg/day, and placebo groups were 73.9, 56.8, and 64.3 %, respectively. Comparison of overall survival among patients classified as Child-Pugh A revealed that survival of the 600 mg/day group (n = 105) was significantly longer than that of the placebo group (n = 108) (hazard ratio 0.575, 95 % CI 0.341–0.967; P = 0.0347).
Conclusions
Administration of 600 mg/day peretinoin to patients with hepatitis C virus-related HCC who have completed curative therapy may improve survival for those classified as Child-Pugh A, for whom liver function is relatively stable.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world, affecting 740,000 people annually [1]. The incidence of HCC has been rising due to an increase in hepatitis C virus (HCV) infections [2–4]. HCC comprises 94 % of all primary liver cancers in Japan; one prominent cause is infection by HCV [4]. Hepatitis C virus-related HCC (HCV-HCC) comprises 75 % of all HCC cases, while HCC due to hepatitis B virus infection (HBV-HCC) comprises 10–15 % [5].
Early diagnosis and curative treatment for HCC, such as hepatectomy or radiofrequency ablation (RFA, a localized therapy), has become more prevalent in recent years. However, high recurrence rates and unfavorable prognoses remain even after curative treatment for HCV-HCC. Cumulative recurrence rates of HCV-HCC are high, with reported rates of 24, 76, and 92 % at 1, 3, and 5 years, respectively, after treatment for the initial onset of liver cancer [6]. Characteristics of HCC recurrence include metastases within the liver, and many patients experience multicentric cancer onset due to underlying liver conditions (e.g., cirrhosis) related to viral liver disease. As underlying liver disease can provide optimal sites for oncogenesis, suppression of multicentric cancer onset is particularly important for improving HCC prognosis, even after curative treatment. Hepatitis virus-related HCC is often treated effectively by antiviral therapies such as interferon [7–9]. However, strategies to control recurrence following cured HCC have not yet been established [10–12].
One method used to control cured HCC recurrence employs a retinoid-based chemoprevention strategy [13]. Peretinoin [(2E,4E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,4,6,10,14-pentaenoic acid] is a retinoid with a vitamin A-like structure discovered by Muto et al. (1980) [14], and currently is one of the potential drugs anticipated to suppress HCC recurrence [15–17].
According to a randomized trial [18], administration of 600 mg/day peretinoin suppressed HCV-HCC recurrence following curative therapy. In Japan, 75 % of patients with HCC test positive for HCV, and compared to other causes of HCC, HCV has the highest risk of recurrence. Thus, the randomized trial aimed to examine the suppressive effects of peretinoin on recurrence among a population with a homogeneously high risk for recurrence, and thus focused on patients who tested positive for HCV following curative therapy. The trial was a multicenter, parallel-group, double-blind, randomized, placebo-controlled trial. Patients who tested positive for HCV (negative for HBV) and underwent liver resection or RFA to treat initial onset or initial recurrence, and who were classified as Child-Pugh A or B, were assigned to one of the following three groups: peretinoin 600 or 300 mg/day, or placebo. The investigational drug was administered orally once a day, for no longer than 2 years. Of the 401 patients registered in the randomized trial between March 2005 and July 2007, 268 were administered the investigational drugs (peretinoin 600 mg/day, n = 134; peretinoin 300 mg/day, n = 134; placebo, n = 133). Our analysis revealed no superiority of the treatment groups (peretinoin 600 and 300 mg/day) over the placebo group with respect to the primary endpoint of recurrence-free survival (P = 0.434). However, the highest 3-year cumulative recurrence-free survival rate (43.7 %) was found in the group administered 600 mg/day peretinoin. The peretinoin 600 mg/day group also showed a significantly lower recurrence rate after 2 years relative to that of the placebo group (hazard ratio 0.27; 95 % CI 0.07–0.96). Sub-group analysis revealed that within the peretinoin 600 mg/day group, the recurrence risk also decreased significantly among those classified as Child-Pugh A (hazard ratio to the placebo group, 0.60; 95 % CI 0.41–0.89) and those with tumor size under 2 cm (hazard ratio to the placebo group, 0.41; 95 % CI 0.23–0.73). Notably, the follow-up duration for the randomized trial was short (median, 2.5 years), and we did not evaluate overall survival among groups.
This study was conducted as an investigator-initiated research, independently of the randomized trial to evaluate the effects of peretinoin on the overall survival up to 6 years of follow-up.
Methods
Study design
This study was a retrospective cohort study which assessed survival outcomes of all participating patients to the randomized trial from the time the trial ended through December 31, 2011. The randomized trial was conducted in accordance with Good Clinical Practice guidelines.
Setting and participants
The principal investigator obtained permission to conduct the study from the ethics committee of Shimonoseki Kohsei Hospital. Approval for data provision was obtained in accordance with the respective institution’s regulations. The study was conducted in compliance with the Declaration of Helsinki, as well as the “Ethical Guidelines for Epidemiological Research” set in Japan by the Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour, and Welfare.
Of the 41 medical institutions where the randomized trial was conducted, we targeted all the institutions that provided written consent to participate in this cohort study and used a data collection form to collect data in the medical records. The collected data included the dates of any deaths up through December 31, 2011 and the last recorded date of survival (in cases where observation was not possible). Data collection forms were collected between January 1, 2012 and December 31, 2012. Data collection forms were mailed from the data center to the medical institutions providing the data. At the medical institutions providing the data, either the physician in charge of the study or the person delegated by this physician provided answers to the questions according to existing resources (medical records, etc.). Anonymized data collection forms were then sent back to the data center.
The primary endpoint was overall survival, defined by the time period beginning on the date of patient registration for the randomized trial until death from all causes or the final date when survival was confirmed. Patients for whom survival was last confirmed before December 2011 were treated as "lost to follow-up."
At the medical institutions providing the data, if a delegate of the physician in charge of the study completed the data collection form, the physician validated the data such that data reliability was guaranteed. The data center conducted an independent and careful review of the collected data according to the data management plan, and then created and maintained the database.
Statistical analysis
In accordance with the principle of the intention-to-treat analysis, this study analyzed data from all patients who had been administered the investigational drug in the randomized trial. Cumulative survival rates in each treatment group were calculated by the Kaplan–Meier method with the dates of each patient registration for the randomized trial as the starting point. The log-rank test was used to compare the 600 mg/day peretinoin and placebo groups, and the 300 mg/day peretinoin and placebo groups. We also calculated hazard ratios and 95 % confidence intervals (CIs) using the Cox proportional hazards model. Two-tailed statistical significance was set at P < 0.05, and P values were not adjusted for multiplicity. All data from December 31, 2011 onwards were treated as censored.
In sub-group analyses, the same survival time analysis method used to evaluate primary endpoints was employed on the classification factors which were set in advance. Classification factors included Child-Pugh classification (A, B), tumor size (<2 cm, ≥2 cm), curative treatment procedure (local ablation, resection), sex (male, female), and age when consent was provided (<65 years, 65–74 years, ≥75 years). All statistical analyses were performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA). This study is registered in the UMIN Clinical Trials Registry (UMIN000006728).
Results
Patients
Survey data were collected from all 392 patients (600 mg/day, n = 132; 300 mg/day, n = 131; placebo, n = 129) (Fig. 1). Patient demographic factors are summarized in Table 1. Sex, age, treatment approach, Child-Pugh classification, and primary tumor size were well balanced among the three treatment groups. Number of deaths in the 600 mg/day group, the 300 mg/day group, and the placebo group were 36, 55, and 47, respectively. In the 600 mg/day group, the 300 mg/day group, and the placebo group, 19, 15, and 15 patients, respectively, were "lost to follow-up."
Flow diagram of patient selection. For the randomized trial, a total of 401 individuals were registered and assigned randomized treatments (peretinoin 600 mg/day, n = 134; peretinoin 300 mg/day, n = 134; placebo, n = 133). Of these, those who were ultimately not administered the investigational drug (2 in the 600 mg/day group, 3 in the 300 mg/day group, and 2 in the placebo group) were excluded, and data from the remaining patients were analyzed (peretinoin 600 mg/day, n = 132; peretinoin 300 mg/day, n = 131; placebo, n = 129)
The median follow-up duration was 1,782 days (4.9 years), with a maximum of 2,364 days (6.5 years). Median and maximum follow-up durations for each of the groups were as follows: 1,815 days (5.0 years) and 2,364 days (6.5 years) for the 600 mg/day group, 1,681 days (4.6 years) and 2,320 days (6.4 years) for the 300 mg/day group, and 1,768 days (4.8 years) and 2,336 days (6.4 years) for the placebo group.
Overall survival
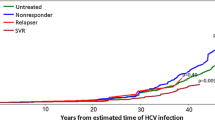
Table 2 shows cumulative survival rates determined using the Kaplan–Meier method. The 2-year survival rates in the 600 mg/day, 300 mg/day, and placebo groups were 93.1, 88.5, and 93.0 %, respectively. The 5-year survival rates in the 600 mg/day, 300 mg/day, and placebo groups were 73.9, 56.8, and 64.3 %, respectively, with the maximum cumulative survival rate observed in the 600 mg/day group. Median survival time in the 300 mg/day and placebo groups were 2,102 days (5.8 years) and 2,165 days (5.9 years), respectively; this parameter could not be calculated for the 600 mg/day group due to good prognosis. Figure 2 shows the Kaplan–Meier curves for the 600 mg/day and placebo groups. With regard to overall survival, no significant difference was observed between the 600 mg/day and placebo groups (hazard ratio 0.726; 95 % CI 0.470–1.122; P = 0.1475 by the log-rank test). Similarly, no significant difference in overall survival was observed between the 300 mg/day and placebo groups (hazard ratio 1.253; 95 % CI 0.849–1.850; P = 0.2547 by the log-rank test).
Sub-group analysis (600 mg/day vs. placebo group)
Results from the sub-group analysis of survival time, which compared the 600 mg/day and placebo groups, are shown in Fig. 3. Factors from the sub-group analysis that were significant at the P < 0.05 level by the log-rank test included the Child-Pugh A classification (hazard ratio 0.575; 95 % CI 0.341–0.967; P = 0.0347 by the log-rank test) and primary tumor size under 2 cm (hazard ratio 0.447; 95 % CI 0.218–0.919; P = 0.0245 by the log-rank test). Kaplan–Meier curves by Child-Pugh A classification for the 600 mg/day and placebo groups (600 mg/day, n = 105; placebo, n = 108) are shown in Fig. 4.
Discussion
Early diagnosis and curative treatment for HCC has become widespread, but following completion of curative treatment for HCV-HCC, recurrence rates remain high and prognosis is poor. The 5-year cumulative survival rate in this cohort study was higher in the 600 mg/day peretinoin group than in the placebo group. Particularly for patients with the Child-Pugh A classification, the 600 mg group had significantly longer survival compared to the placebo group. We believe that this finding will contribute to future attempts for developing measures to prevent HCC recurrence.
The guidelines for clinical studies of HCC [19] recommend that only patients with the Child-Pugh A classification be incorporated into clinical studies, because death due to cirrhosis among patients classified as Child-Pugh B or Child-Pugh C could mask treatment effects. In this study, roughly 80 % of our patients were classified as Child-Pugh A, and thus our results relating to this group may be generalizable. In addition, the results of this study, expressed in patients with tumor size of under 2 cm, are consistent with the report of Nakashima et al. [20] that suggests the incidence of multicentric recurrence is high amongst patients with tumor size of under 2 cm, and peretinoin is particularly effective at suppressing multicentric recurrence in such cases.
From the results of this study and the randomized trial, we speculate that peretinoin improves patient survival time by suppressing HCC recurrence. Among those classified as Child-Pugh A, the hazard ratio of recurrence-free survival for peretinoin and placebo in the randomized trial was 0.60 (95 % CI 0.41–0.89) [18], and the hazard ratio of overall survival for peretinoin 600 mg/day and placebo was 0.575 (95 % CI 0.341–0.967). The similar hazard ratios could be explained by the following reasons. First, the investigational drug was administered for 2 years maximum in the randomized trial, and the median follow-up duration of this cohort study was nearly 5 years. Peretinoin is particularly well-known for its capacity to suppress multicentric recurrence, so given that a certain duration of time is required for a pre-cancerous lesion to develop into cancer, we surmise that peretinoin may have continued to work effectively in suppressing recurrence even after completion of peretinoin treatment. A follow-up survey [21] of the randomized trial conducted by Muto et al. [16] found that after administering this drug for 1 year, continued effects were noted for at least 150 weeks (roughly 3 years) following treatment completion. In addition, peretinoin has been found to suppress platelet-derived growth factor C [22, 23], and thus it may be continuously suppressing recurrence by inhibiting the progression of hepatic fibrosis.
The limitation of this study was that the randomized trial was designed to evaluate recurrence-free survival in peretinoin-treated patients, and thus the number of patients may have been insufficient for evaluating the primary endpoint of this cohort study, i.e., overall survival. However, being able to collect and evaluate data from all patients who were administered the investigational drug in the randomized trial improved the statistical accuracy of our analysis.
Despite the limitation, the important point of this study is that by focusing on survival versus death, which is an index with no room for subjectivity, we were able to determine that among those classified as Child-Pugh A, the 600 mg/day group had a significantly longer overall survival compared to the placebo group. Notably, independent of this cohort study, a phase III trial is currently underway to examine the effects of peretinoin on controlling HCC recurrence among those classified as Child-Pugh A who have completed curative treatment for HCV-HCC. In conclusion, administration of 600 mg/day peretinoin to patients who have completed curative treatment for HCV-HCC is anticipated to improve survival of patients with relatively stable liver function, such as those classified as Child-Pugh A. Our finding provides novel insights into the understanding of 5-year survival after treatment with investigational drug in patients with HCV-HCC.
References
Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: gLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91.
Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14.
El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–23.
Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–64.
Shiratori Y, Shiina S, Teratani T, et al. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138:299–306.
Ikeda K, Arase Y, Saitoh S, et al. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology. 2000;32:228–32.
Suou T, Mitsuda A, Koda M, et al. Interferon alpha inhibits intrahepatic recurrence in hepatocellular carcinoma with chronic hepatitis C: a pilot study. Hepatol Res. 2001;20:301–11.
Kubo S, Nishiguchi S, Hirohashi K, et al. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. A randomized, controlled trial. Ann Intern Med. 2001;134:963–7.
Breitenstein S, Dimitroulis D, Petrowsky H, et al. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg. 2009;96:975–81.
Mathurin P, Raynard B, Dharancy S, et al. Meta-analysis: evaluation of adjuvant therapy after curative liver resection for hepatocellular carcinoma. Aliment Pharmacol Ther. 2003;17:1247–61.
Samuel M, Chow PK, Chan Shih-Yen E, et al. Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. Cochrane Database Syst Rev. 2009;1:CD001199.
Sporn MB, Newton DL. Chemoprevention of cancer with retinoids. Fed Proc. 1979;38:2528–34.
Araki H, Shidoji Y, Yamada Y, et al. Retinoid agonist activities of synthetic geranyl geranoic acid derivatives. Biochem Biophys Res Commun. 1995;209:66–72.
Muto Y, Moriwaki H, Ninomiya M, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561–7.
Muto Y, Moriwaki H, Saito A. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. N Engl J Med. 1999;340:1046–7.
Okusaka T, Ueno H, Ikeda M, et al. Phase I and pharmacokinetic clinical trial of oral administration of the acyclic retinoid NIK-333. Hepatol Res. 2011;41:542–52.
Okita K, Izumi N, Matsui O, et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol. 2014;. doi:10.1007/s00535-014-0956-9.
Llovet JM, Di Bisceglie AM, Bruix J, et al. Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711.
Nakashima Y, Nakashima O, Tanaka M, et al. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res. 2003;26:142–7.
Takai K, Okuno M, Yasuda I, et al. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. Updated analysis of the long-term follow-up data. Intervirology. 2005;48:39–45.
Okada H, Honda M, Campbell JS, et al. Acyclic retinoid targets platelet-derived growth factor signaling in the prevention of hepatic fibrosis and hepatocellular carcinoma development. Cancer Res. 2012;72:4459–71.
Honda M, Yamashita T, Arai K, et al. Peretinoin, an acyclic retinoid, improves the hepatic gene signature of chronic hepatitis C following curative therapy of hepatocellular carcinoma. BMC Cancer. 2013;. doi:10.1186/1471-2407-13-191.
Acknowledgments
The authors would like to thank all patients and their families who participated in this study. We also thank all who were involved in the research and would like to acknowledge Non-Profit Organization Japan Clinical Research Support Unit for their assistance in data management and statistical analyses, and Statcom Co., Ltd. for their assistance in preparing and editing this manuscript. This study was presented in part at the seventh annual conference of the International Liver Cancer Association, Washington, D.C., September 13-15, 2013. This study was funded by the Comprehensive Support Project (CSP) of the Public Health Research Foundation. The corporate and individual sponsors of this study are listed on the CSPOR website (http://www.csp.or.jp/cspor/kyousan_e.html). The pharmaceutical manufacturer/distributor who provided financial contribution as a corporate sponsor took no part in this study other than providing information relevant to proper use of the study drug(s). The following institutions provided data: Ehime University Hospital, Fukuoka University Hospital, Gifu Municipal Hospital, Gifu University Hospital, Hiroshima Prefectural Hospital, Hiroshima University Hospital, Hokkaido University Hospital, Hyogo College of Medicine, Iwate Medical University Hospital, Jichi Medical University Hospital, Kagoshima University Medical And Dental Hospital, Kansai Medical University Takii Hospital, Kinki University Hospital, Kitano Hospital, Kitasato University East Hospital, Kokura Memorial Hospital, Kumamoto University Hospital, Musashino Red Cross Hospital, Nagasaki University Hospital, National Cancer Center Hospital, National Cancer Center Hospital East, National Hospital Organization Nagasaki Medical Center, Oita University Hospital, Osaka City General Hospital, Osaka City University Hospital, Osaka General Medical Center, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka Red Cross Hospital, Osaka Rosai Hospital, Saiseikai Fukuoka General Hospital, Sapporo Kosei General Hospital, Shimonoseki Kosei Hospital, Showa University Hospital, Teine-Keijinkai Hospital, The University of Tokyo Hospital, Tokyo Medical And Dental University Hospital Faculty of Medicine, Tokyo Medical University Hospital, Toranomon Hospital, Toyohashi Municipal Hospital, Yamaguchi University Hospital and Yokohama City University Medical Center.
Conflict of interest
Okita has received lecture fees from Kowa. Izumi has received lecture fees from MSD, Chugai Pharmaceutical, Daiichi Sankyo, and Bayer Yakuhin. Masafumi Ikeda has received research funding from Kowa. Kokudo has received research funding from Dainippon Sumitomo Pharma, Bayer Yakuhin, Merk Serono, Bristol-Myers Squibb, Chugai Pharmaceutical, Taiho Pharmaceutical, and Yakult Pharmaceutical. Ueshima has received lecture fees from Bayer Yakuhin, MSD, Ajinomoto Pharmaceuticals, Eisai, Dainippon Sumitomo Pharma, Eidia, Takeda Pharmaceutical, Janssen Pharmaceutical, Daiichi Sankyo, and Boehringer Ingelheim Japan. Kudo has received lecture fees from Bayer Yakuhin and Eisai. Okusaka has received research funding from Kowa. Ohashi received executive salaries from Statcom, lecture fees from Chugai Pharmaceutical and Shionogi, manuscript fee from DNP Media Create, and research funding from Kowa Pharmaceutical, Astellas Pharma, Takeda Pharmaceutical, and Kyowa Hakko Kirin. Kumada holds a patent on SRL, and has received lecture fees from MSD, Bristol-Myers Squibb, Mitsubishi Tanabe Pharma, Dainippon Sumitomo Pharma, Toray Industries, and Ajinomoto Pharmaceuticals. The other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
UMIN clinical trial registration number: UMIN000006728.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Okita, K., Izumi, N., Ikeda, K. et al. Survey of survival among patients with hepatitis C virus-related hepatocellular carcinoma treated with peretinoin, an acyclic retinoid, after the completion of a randomized, placebo-controlled trial. J Gastroenterol 50, 667–674 (2015). https://doi.org/10.1007/s00535-014-0996-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-014-0996-1







 Age at the time of registration to the randomized trial.
Age at the time of registration to the randomized trial.  Log-rank test
Log-rank test