Abstract
Background
Oxaliplatin-induced peripheral neuropathy (OIPN) is a common and dose-limiting toxicity that markedly limits the use of oxaliplatin and affects quality of life. Statins have been shown to exert neuroprotective effects in preclinical settings. The aim of the present study was to clarify whether statins prevented OIPN in patients with colorectal cancer (CRC) receiving adjuvant CAPOX therapy.
Methods
We examined 224 patients who received adjuvant CAPOX therapy for CRC between July 2010 and December 2021 at our hospital. Patients were divided into “Statin” and “Non-statin” groups based on statin use. Details on and the adverse events of adjuvant CAPOX therapy were examined in association with statin use.
Results
Thirty-one patients (14%) were treated with statins. There were no intergroup differences in the relative dose intensity or number of CAPOX cycles between the Statin and Non-statin groups. In total, 94% of patients in the Statin group and 95% of those in the Non-statin group developed OIPN (p=0.67). The severity of OIPN was similar between the two groups (p=0.89). The frequency of treatment delays in CAPOX did not significantly differ between the Statin and Non-statin groups (16% vs. 11%, p=0.45).
Conclusions
The efficacy of statins to attenuate OIPN during adjuvant CAPOX therapy was not apparent in the current study. Further studies are needed to confirm the present results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most deadly and fourth most commonly diagnosed cancer worldwide [1, 2]. Previous randomized clinical trials (RCTs) revealed that 5-fluorouracil (5-FU) effectively prevented the recurrence of CRC after radical resection [3,4,5]. In another series of RCTs, better survival was achieved by oxaliplatin in combination with 5-FU than by 5-FU alone for stage II and III colon cancer [6,7,8]. Therefore, a growing number of locally advanced CRC patients have received oxaliplatin-based adjuvant chemotherapy [9,10,11].
Major adverse events of oxaliplatin include peripheral neuropathy, myelosuppression, and gastrointestinal reactions, such as diarrhea and stomatitis [12, 13]. These unfavorable effects of oxaliplatin may disrupt the treatment plan and reduce the drug compliance of CRC patients. Oxaliplatin-induced peripheral neuropathy (OIPN) leads to drug reductions or discontinuation and impairs the quality of life of patients [14, 15]. Despite intense preclinical and clinical research, no drugs have been recommended to prevent the development of OIPN [16].
Animal studies previously demonstrated that statins, HMG-CoA reductase inhibitors, exerted neuroprotective effects by attenuating oxidative stress [17, 18]. However, clinical studies reported conflicting findings; a RCT showed that rosuvastatin ameliorated diabetic polyneuropathy [19], whereas a prospective cohort study revealed that reductions in cholesterol levels increased the rate of painful neuropathic syndromes [20]. Therefore, the efficacy of statins to prevent neuropathy in humans remains unclear.
Regarding the relationship between statins and OIPN, only one retrospective study reported that the incidence of OIPN decreased with statin use in patients with several cancer types [21]. Therefore, we herein investigated whether statins prevented OIPN in CRC patients receiving capecitabine and oxaliplatin (CAPOX) therapy.
Materials and methods
Patients
We investigated consecutive Japanese patients who underwent radical surgery for primary CRC and received adjuvant CAPOX therapy between July 2010 and December 2021 at the University of Tokyo Hospital. Patients who received preoperative chemoradiotherapy without oxaliplatin were included. We excluded patients who had previously received duloxetine, a medication recommended for the treatment of OIPN [9, 10].
The present retrospective study was approved by the Ethics Committee of the University of Tokyo (No. 3252-[16]).
Adjuvant CAPOX therapy
At our hospital, we recommend adjuvant chemotherapy to CRC patients based on the latest guidelines of the Japanese Society for Cancer of the Colon and Rectum [22]. However, chemotherapy regimens are modified at the physician’s discretion according to the patients’ age, performance status (PS), and other comorbidities. CAPOX therapy consisted of the intravenous infusion of 130 mg/m2 oxaliplatin and the oral administration of capecitabine at a dose of 1,000 mg/m2 twice daily for two weeks. The treatment course was repeated every three weeks [23].
Data extraction
We retrieved the following data from our prospective database and patient medical charts: age, sex, body mass index, Eastern Cooperative Oncology Group PS, comorbidities, such as diabetes mellitus, cardiac, pulmonary, renal, and hepatic diseases, statin use, the primary location, the pathological classification of tumors according to the American Joint Committee on Cancer staging manual [24], the relative dose intensities of chemotherapeutic drugs, the number of CAPOX cycles, dose reductions, unscheduled treatment delays, and adverse events graded according to Common Terminology Criteria for Adverse Events version 5.0 [25].
We divided patients into two groups according to statin use: the ‘Statin’ and “Non-statin” groups.
Statistical analysis
Statistical analyses were performed using JMP Pro 16.2.0 (SAS Institute, Cary, NC, USA). All variables were summarized as medians (ranges), means ± standard deviations, or numbers (percentages). Quantitative variables were compared using the Mann-Whitney U test. Qualitative variables were compared using Fisher’s exact test or the chi-squared test with Yates’ correction where appropriate. All reported p-values were two-sided, and results were considered to be significant when p-values were <0.05.
Results
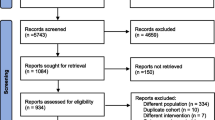
A total of 224 patients who received CAPOX therapy were included in the present study. Thirty-one patients (14%) received statins during adjuvant CAPOX therapy (Fig. 1). Statins used in the Statin group are shown in Table 1.
Table 2 summarizes the characteristics of patients according to statin use. Patients in the Statin group were older than those in the Non-statin group (69 vs. 56 years old, p<0.001). Body mass index was higher in the Statin group than in the Non-statin group (p=0.023). In addition, the Statin group included more patients with diabetes mellitus, hypertension, cardiovascular disease, and dyslipidemia than the Non-statin group (p=0.008, p=0.019, p<0.001, and p<0.001, respectively). No significant differences were observed in other parameters between the two groups.
The treatment details of adjuvant CAPOX therapy are reviewed in Table 3. There were no significant differences in the relative dose intensity or number of cycles between the Statin and Non-statin groups.
Table 4 shows comparative adverse events during CAPOX according to statin use. In total, 94% of patients in the Statin group and 95% in the Non-statin group exhibited OIPN of any grade (p=0.67); both groups showed a similar distribution of the severity of OIPN (p=0.89). Moreover, grade ≥2 OIPN often occurred as early as at the end of the second cycle of CAPOX regardless of statin use (p=0.99). The incidence of dose reductions or treatment delays in CAPOX due to OIPN did not significantly differ between the Statin and Non-statin groups (13% vs. 12%, p=1.0 and 16% vs. 11%, p=0.45, respectively). The overall incidence of grade 3/4 adverse events other than OIPN was 23% in the Statin group and 30% in the Non-statin group (p=0.43).
Discussion
Although OIPN is a major dose-limiting adverse event of oxaliplatin, there are currently few preventive or treatment measures. Animal models of chemotherapy-induced peripheral neuropathy, including OIPN, have been established since the late 2000s [26,27,28], and many drugs have been examined as potential medications [29]. Preclinical studies previously suggested the neuroprotective effects of statins [17, 18, 30, 31]. However, only one study investigated the relationship between statins and OIPN in clinical settings, and subjects (277 patients) received different dose intensities of oxaliplatin with various drug combinations [21]. To the best of our knowledge, this is the first study to investigate the efficacy by which statins prevent OIPN during CAPOX therapy in CRC patients. In our cohort of 224 patients, we did not detect any relationships between statin use and the incidence or severity of OIPN.
Regarding the mechanisms of OIPN, previous studies suggested that OIPN is caused by ion channel dysfunction, glial activation, nuclear DNA damage, mitochondrial damage, neuroinflammation, and oxidative stress [16, 32]. Among these factors, statins were shown to have the potential to attenuate oxidative damage; two research groups reported that statins exerted neuroprotective effects through the suppression of superoxide formation in preclinical models [30, 31]. In 2022, a new study indicated that statins prevented OPIN in a rat model by inducing Gstm1 mRNA, which promotes the detoxification of reactive oxygen species [21].
Despite promising findings from preclinical studies, clinical evidence suggested that the administration of statins to patients was associated with the development of neuropathy [33, 34]. The reasons for these discrepancies remain unclear; however, a narrative review on statins and neuropathic pain reported that reductions in low-density lipoproteins by statins may prevent the delivery of vitamin E, an essential factor for supporting healthy neural tissues [35]. A deficiency in vitamin E may disrupt nerve fibers, fat metabolism, and mitochondrial transport, which collectively result in increased neuropathic pain [35]. Further studies are needed to elucidate the complex relationship between statins and neuropathy.
Previous studies reported that OIPN occurred in 80–95% patients after treatment with oxaliplatin [36,37,38]. Similarly, in our Japanese cohort, 94% of patients receiving statins and 94% without statins developed OIPN. However, in the study by Zamami et al., only 65% of Japanese statin users developed OIPN during oxaliplatin-based therapy [21]. These inconsistent findings may be attributed to differences in patient backgrounds; we only included CRC patients, while Zamami et al. examined patients with various cancer types [21]. Although treatment details were not reported in that study, the intensity or dosing frequency of oxaliplatin may have differed between their study and ours.
The relationship between OIPN and diabetes mellitus currently remains unclear; however, a few previous studies suggested that patients with diabetes mellitus were more likely to develop early-onset and persistent OIPN than non-diabetic patients [39,40,41]. In the present study, the Statin group comprised more patients with diabetes mellitus than the Non-statin group (29% vs. 11%). This imbalance may have contributed to the similar incidence and severity of OIPN between the two groups, even if statins protect against neuropathy.
There are several limitations that need to be addressed. This was a retrospective study conducted at a single hospital. In addition, the number of patients receiving statins was relatively small, which may have caused type II errors. Besides diabetes mellitus, the intergroup disparities in age, body mass index, hypertension, cardiovascular disease and dyslipidemia may have contributed to the similar incidence and severity of OIPN between the two groups, even if statins protect against neuropathy. Moreover, the present study may have included a selection bias; adjuvant chemotherapy may not have been selected for patients who already had neuropathy and patients with dyslipidemia who were likely to have severe concurrent comorbidities. We also included some patients with a short follow-up, which may hamper the evaluation of long-term changes in chronic OIPN. Furthermore, the duration of and adherence to statin therapy were not examined.
Conclusions
In CAPOX therapy for CRC patients, the effectiveness of statins for reducing the incidence or severity of OIPN was not observed in our cohort. The frequency of dose reductions or treatment delays was also independent of statin use. Further studies with a larger patient cohort are needed to confirm the present results.
Data availability
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Rawla P, Sunkara T, Barsouk A (2019) Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol 14(2):89–103
Laurie JA, Moertel CG, Fleming TR et al (1989) Surgical adjuvant therapy of largebowel carcinoma : an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol 7(10):1447–1456
Moertel CG, Fleming TR, Macdonald JS et al (1990) Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322:352–358
Moertel CG, Fleming TR, Macdonald JS et al (1995) Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma : a final report. Ann Intern Med 122:321–326
André T, Boni C, Mounedji-Boudiaf L et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350(23):2343–2351
Haller DG, Tabernero J, Maroun J et al (2015) Capecitabine Plus Oxaliplatin Compared With Fluorouracil/Folinic Acid As Adjuvant Therapy for Stage III Colon Cancer: Final Results of the NO16968 Randomized Controlled Phase III Trial. J Clin Oncol 33(32):3733–3740
Kuebler JP, Wieand HS, O’Connell MJ et al (2007) Oxaliplatin Combined With Weekly Bolus Fluorouracil and Leucovorin As Surgical Adjuvant Chemotherapy for Stage II and III Colon Cancer: Results From NSABP C-07. J Clin Oncol 25(16):2198–2204
National Comprehensive Cancer Network (2022) Clinical Practice Guidelines in Oncology. Colon Cancer, version 2.2022. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed November 2022
National Comprehensive Cancer Network (2022) Clinical Practice Guidelines in Oncology. Rectal Cancer, version 3.2022. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed November 2022
Osumi H, Shinozaki E, Suenaga M et al (2017) Change in clinical outcomes during the transition of adjuvant chemotherapy for stage III colorectal cancer. PLoS One 12(5):e0176745
Andre T, Boni C, Navarro M et al (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adju-vant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27(19):3109–3116
Cassidy J, Misset JL (2002) Oxaliplatin-related side effects: characteristics and management. Semin Oncol 29(5):11–20
Han CH, Kilfoyle DH, Hill AG et al (2016) Preventing oxaliplatin-induced neurotoxicity: rationale and design of phase Ib randomized, double-blind, placebo-controlled, cross-over trials for early clinical evaluation of investigational therapeutics. Expert Opin Drug Metab Toxicol 12(12):1479–1490
Prutianu I, Alexa-Stratulat T, Cristea EO et al (2022) Oxaliplatin-induced neuropathy and colo-rectal cancer patient's quality of life: Practical lessons from a prospective cross-sectional, real-world study. World J Clin Cases 10(10):3101–3112
Wei G, Gu Z, Gu J et al (2021) Platinum accumulation in oxaliplatin-induced peripheral neuropathy. J Peripher Nerv Syst 26(1):35–42
Pathak NN, Balaganur V, Lingaraju MC et al (2014) Atorvastatin attenuates neuropathic pain in ratneuropathy model by down-regulating oxidative damage at peripheral, spinal and supraspinal levels. Neurochem Int 68:1–9
Jabeen A, Khan UA, Ayub M, Hameed MA (2011) Effects of simvastatin and alpha-tocopherol on disturbed nerve conduction in obese Sprague Dawley rats. J Ayub Med Coll Abbottabad 23(3):18–22
Hernandez-Ojeda J, Roman-Pintos LM, Rodriguez-Carrizalez AD et al (2014) Effect of rosuvastatin on dia-betic polyneuropathy: a randomized, double-blind, placebo-controlled Phase IIa study. Diabetes Metab Syndr Obes 7:401–407
Jende JME, Groener JB, Rother C et al (2019) Associationof serum cholesterol levels with peripheral nerve damage in patients with type 2 diabetes. JAMA Netw Open 2(5):e194798
Zamami Y, Niimura T, Kawashiri T et al (2022) Identification of prophylactic drugs for oxaliplatin-induced peripheral neuropathy using big data. Biomed Pharmacother 148:112744
Hashiguchi Y, Muro K, Saito Y et al (2020) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25(1):1–42
Schmoll HJ, Cartwright T, Tabernero J et al (2007) Phase III Trial of Capecitabine Plus Oxaliplatin As AdjuvantTherapy for Stage III Colon Cancer: A Planned SafetyAnalysis in 1,864 Patients. J Clin Oncol 25(1):102–109
Amin MB, Edge S, Greene F et al (2017) AJCC Cancer Staging Manual, 8th edn. Springer, NewYork (NY)
US Department of Health and Human Services (2017) Common Terminology Criteria for Adverse Events (CTCAE). version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed March 2023
Ling B, Authier N, Balayssac D et al (2007) Behavioral and pharmacological description of oxaliplatin-induced painful neuropathy in rat. Pain 128(3):225–234
Ta LE, Low PA, Windebank AJ (2009) Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol Pain 5:9
Sakurai M, Egashira N, Kawashiri T et al (2009) Oxaliplatin-induced neuropathy in the rat: Involvement ofoxalate in cold hyperalgesia but not mechanical allodynia. Pain 147(1-3):165–174
Kawashiri T, Mine K, Kobayashi D et al (2021) Therapeutic Agents for Oxaliplatin-Induced Peripheral Neuropathy; Experimental and Clinical Evidence. Int J Mol Sci 22(3):1393
Uekawa K, Hasegawa Y, Ma M et al (2014) Rosuvastatin ameliorates early brain injury after subarachnoid hemorrhage via suppression of superoxide formation and nuclear factor-kappa B activation in rats. J Stroke Cerebrovasc Dis 23(6):1429–1439
Rayegan S, Dehpour AR, Sharifi AM (2017) Studying neuroprotective effect of Atorvastatin as a small molecule drug on high glucose-induced neurotoxicity in undifferentiated PC12 cells: role of NADPH oxidase. Metab Brain Dis 32(1):41–49
Kang L, Tian Y, Xu S et al (2021) Oxaliplatin-induced peripheral neuropathy: clinical features, mechanisms, prevention and treatment. J Neurol 268(9):3269–3282
Chong PH, Boskovich A, Stevkovic N et al (2004) Statin-associated peripheral neuropathy: review of the literature. Pharmacotherapy 24(9):1194–1203
Bhalla S, Singh N, Jaggi AS (2014) Statins: do they aggravate or ameliorate neuropathic pain? J Pain 15(11):1069–1080
Pergolizzi JV Jr, Magnusson P, LeQuang JA et al (2020) Statins and Neuropathic Pain: A Narrative Review. Pain Ther 9(1):97–111
Han CH, Kilfoyle DH, Hill AG et al (2016) Preventing oxali-platin-induced neurotoxicity: rationale and design of phase Ib randomized, double-blind, placebo-controlled, cross-over tri-als for early clinical evaluation of investigational therapeutics. Expert Opin Drug Metab Toxicol 12(12):1479–1490
Jamie RB, Gladys M, Eileen MD et al (2016) Chemotherapy-induced peripheral neuropathy: current status and progress. Gynecol Oncol 140(1):176–183
Ewertz M, Qvortrup C, Eckhoff L (2015) Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol 54(5):587–591
Sempere-Bigorra M, Julián-Rochina I, Cauli O (2021) Chemotherapy-Induced Neuropathy and Diabetes: A Scoping Review. Curr Oncol 28(4):3124–3138
Lee S, Ma C, Shi Q et al (2023) Potential Mediators of Oxaliplatin-Induced Peripheral Neuropathy From Adjuvant Therapy in Stage III Colon Cancer: Findings From CALGB (Alliance)/SWOG 80702. J Clin Oncol 41(5):1079–1091
Ottaiano A, Nappi A, Tafuto S et al (2016) Diabetes and Body Mass Index Are Associated with Neuropathy and Prognosis in Colon Cancer Patients Treated with Capecitabine and Oxaliplatin Adjuvant Chemotherapy. Oncology 90(1):36–42
Funding
Open access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
Kazuaki Okamoto, Hiroaki Nozawa and Soichiro Ishihara developed the study design and concept, retrieved the data of patients and carried out the analysis. Kazuaki Okamoto, Hiroaki Nozawa, Shigenobu Emoto, Koji Murono, Kazuhito Sasaki and Soichiro Ishihara participated in writing and revising the manuscript critically. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committees of the University of Tokyo (No. 3252-[16]).
Informed consent
All patients were given informed consent before enrollment.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okamoto, K., Nozawa, H., Emoto, S. et al. Does statin suppress oxaliplatin-induced peripheral neuropathy in patients with colorectal cancer? A single-center observational study. Support Care Cancer 31, 660 (2023). https://doi.org/10.1007/s00520-023-08134-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08134-2