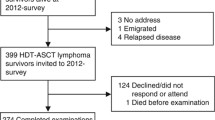
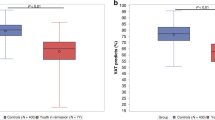
Abstract
Purpose
Childhood lymphoma survivors (CLSs) are at high risk of reduced daily activity. This work studied metabolic substrate use and cardiorespiratory function in response to exercise in CLSs.
Methods
Twenty CLSs and 20 healthy adult controls matched for sex, age, and BMI took an incremental submaximal exercise test to determine fat/carbohydrate oxidation rates. Resting echocardiography and pulmonary functional tests were performed. Physical activity level, and blood metabolic and hormonal levels were measured.
Results
CLSs reported more physical activity than controls (6317 ± 3815 vs. 4268 ± 4354 MET-minutes/week, p = 0.013), had higher resting heart rate (83 ± 14 vs. 71 ± 13 bpm, p = 0.006), and showed altered global longitudinal strain (− 17.5 ± 2.1 vs. − 19.8 ± 1.6%, p = 0.003). We observed no difference in maximal fat oxidation between the groups, but it was reached at lower relative exercise intensities in CLSs (Fatmax 17.4 ± 6.0 vs. 20.1 ± 4.1 mL/kg, p = 0.021). At V̇O2 peak, CLSs developed lower relative exercise power (3.2 ± 0.9 vs. 4.0 ± 0.7 W/kg, p = 0.012).
Conclusion
CLSs reported higher levels of physical activity but they attained maximal fat oxidation at lower relative oxygen uptake and applied lower relative power at V̇O2 peak. CLSs may thus have lower muscular efficiency, causing greater fatigability in response to exercise, possibly related to chemotherapy exposure during adolescence and childhood. Long-term follow-up is essential and regular physical activity needs to be sustained.


Similar content being viewed by others
Data Availability
Data are available on request.
Abbreviations
- 3MST :
-
3-Minute step test
- BMI:
-
Body mass index
- CHOs:
-
Carbohydrates
- CLSs:
-
Childhood lymphoma survivors
- CRP:
-
C reactive protein
- ES:
-
Effect size
- FEV1 :
-
Forced expiratory volume in one second
- FVC:
-
Forced vital capacity
- GH:
-
Growth hormone
- GLS :
-
Global longitudinal strain
- HOMA-IR:
-
Homeostasis
- HR:
-
Heart rate
- IWST:
-
Isometric wall squat test
- IGF-1:
-
Insulin-like growth factor
- MFO:
-
Maximal fat oxidation
- NT-pro-BNP:
-
N-terminal section of brain natriuretic peptide precursor
- PA:
-
Physical activity
- Q1:
-
First quartile
- Q3:
-
Third quartile
- RER:
-
Respiratory exchange ratio
- RV:
-
Residual volume
- SEV:
-
Systolic ejection volume
- TLC:
-
Total lung capacity
- TLCO:
-
Lung diffusion capacity for carbon monoxide
- V̇CO2 :
-
Carbon dioxide excretion
- V̇O2 :
-
Dioxygen uptake
References
Wogksch MD, Howell CR, Wilson CL et al (2019) Physical fitness in survivors of childhood Hodgkin lymphoma: a report from the St. Jude Lifetime Cohort. Pediatr Blood Cancer 66:e27506
Chueh HW, Yoo JH (2017) Metabolic syndrome induced by anticancer treatment in childhood cancer survivors. Ann Pediatr Endocrinol Metab 22:82
Sorensen JC, Cheregi BD, Timpani CA et al (2016) Mitochondria: inadvertent targets in chemotherapy-induced skeletal muscle toxicity and wasting? Cancer Chemother Pharmacol 78:673–683
Gavotto A, Requirand A, Amedro P (2021) Épreuve d’effort cardio-respiratoire chez l’enfant. Perfect En Pédiatrie 4:144–151
Guler E, Col N, Buyukcelik M et al (2018) Prevalence of hypertension determined by ambulatory blood pressure monitoring (ABPM) and body composition in long-term survivors of childhood cancer. Pediatr Hematol Oncol 35:1–10
Bhakta N, Liu Q, Ness KK et al (2017) The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet Lond Engl 390:2569–2582
Ness KK, Mertens AC, Hudson MM et al (2005) Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med 143:639–647
Hess SL, Jóhannsdóttir IM, Hamre H et al (2011) Adult survivors of childhood malignant lymphoma are not aware of their risk of late effects. Acta Oncol Stockh Swed 50:653–659
Ruiz JR, Castro-Pinero J, Artero EG et al (2009) Predictive validity of health-related fitness in youth: a systematic review. Br J Sports Med 43:909–923
Brun J-F, Varlet-Marie E, Romain A-J et al (2011) Interrelationships among body composition, blood rheology and exercise performance. Clin Hemorheol Microcirc 49:183–197
Riddell MC, Bar-Or O, Wilk B et al (1985) Substrate utilization during exercise with glucose and glucose plus fructose ingestion in boys ages 10–14 yr. J Appl Physiol Bethesda Md 2001(90):903–911
Pegon C, Rochette E, Rouel N et al (2020) Childhood leukemia survivors and metabolic response to exercise: a pilot controlled study. J Clin Med 9:E562
Yadav A, Kataria MA, Saini V et al (2013) Role of leptin and adiponectin in insulin resistance. Clin Chim Acta Int J Clin Chem 417:80–84
Diniz MDFHS, Beleigoli AMR, Schmidt MI et al (2020) Homeostasis model assessment of insulin resistance (HOMA-IR) and metabolic syndrome at baseline of a multicentric Brazilian cohort: ELSA-Brasil study. Cad Saude Publica 36:e00072120
Barbosa-Cortés L, López-Alarcón M, Mejía-Aranguré JM et al (2017) Adipokines, insulin resistance, and adiposity as a predictors of metabolic syndrome in child survivors of lymphoma and acute lymphoblastic leukemia of a developing country. BMC Cancer 17:125
Armstrong T, Bull F (2006) Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J Public Health 14:66–70
Ainsworth BE, Haskell WL, Whitt MC et al (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32:S498-516
Iturain Barrón A, Quintana Riera S, Reychler G (2021) The 3 Minute Step Test is a validated field test to evaluate the functional exercise capacity in children aged 6 to 12. Respir Med Res 80:100833
Lea JWD, O’Driscoll JM, Coleman DA et al (2021) Validity and reliability of the ‘Isometric Exercise Scale’ (IES) for measuring ratings of perceived exertion during continuous isometric exercise. Sci Rep 11:5334
Goodpaster BH (2002) Measuring body fat distribution and content in humans. Curr Opin Clin Nutr Metab Care 5:481–487
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J - Cardiovasc Imaging 16:233–271
Compher C, Frankenfield D, Keim N et al (2006) Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc 106:881–903
Calcium I of M (US) C to RDRI for VD and, Ross AC, Taylor CL, et al. - Dietary reference intakes for calcium and vitamin D - NCBI bookshelf. Available at: http://www.ncbi.nlm.nih.gov/books/NBK56068/table/summarytables.t3/. AccessedFebruary 12, 2020.
Dafoe W (2007) Principles of exercise testing and interpretation. Can J Cardiol 23:274
Riddell MC (1985) The endocrine response and substrate utilization during exercise in children and adolescents. J Appl Physiol Bethesda Md 2008(105):725–733
Frayn KN (1988) Indirect calorimetry. Metabolism 37:1185
Fel S, Rochette E, Walther G et al (2021) Maximal fat oxidation during exercise is already impaired in pre-pubescent children with type 1 diabetes mellitus. Front Physiol 12:664211
Brun JF, Varlet-Marie E, Romain AJ, Mercier J (2011) Measurement and physiological relevance of the maximal lipid oxidation rate during exercise (LIPOXmax). INTECH book. Sports Medicine and Sports Injuries
Nikolovski Z, Barbaresi S, Cable T et al (2021) Evaluating the influence of differences in methodological approach on metabolic thresholds and fat oxidation points relationship. Eur J Sport Sci 21:61–68
Bozzetti F (2020) Chemotherapy-induced sarcopenia. Curr Treat Options Oncol 21:7
Lanfranconi F, Pollastri L, Ferri A et al (2014) Near infrared spectroscopy (NIRS) as a new non-invasive tool to detect oxidative skeletal muscle impairment in children survived to acute lymphoblastic leukaemia. PLoS ONE 9:e99282
Teng AE, Noor B, Ajijola OA et al (2021) Chemotherapy and radiation-associated cardiac autonomic dysfunction. Curr Oncol Rep 23:14
Yang H, Wright L, Negishi T et al (2018) Research to practice: assessment of left ventricular global longitudinal strain for surveillance of cancer chemotherapeutic-related cardiac dysfunction. JACC Cardiovasc Imaging 11:1196–1201
de la Fuente A, Santisteban M, Lupón J et al (2022) A fibrosis biomarker early predicts cardiotoxicity due to anthracycline-based breast cancer chemotherapy. Cancers 14:2941
Cornelissen VA, Verheyden B, Aubert AE et al (2010) Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J Hum Hypertens 24:175–182
Ring-Dimitriou S, Paulweber B, von Duvillard SP et al (2006) The effect of physical activity and physical fitness on plasma adiponectin in adults with predisposition to metabolic syndrome. Eur J Appl Physiol 98:472–481
Maunder E, Plews DJ, Kilding AE (2018) Contextualising maximal fat oxidation during exercise: determinants and normative values. Front Physiol 9:599
Acknowledgements
This project was conducted as part of a research program by the Health and Exercise Response in Children with Chronic and auto-immUne pathoLogiEs (HERCCULE). The investigators were Alexandre Armand; Corinne Borderon; Aurélie Chausset; Pascale Duché; Stéphane Echaubard; Solenne Fel; Justyna Kanold; Emmanuelle Labraise; Etienne Merlin; Stéphane Nottin; Justine Paysal; Charline Pegon; Bruno Pereira; Ruddy Richard; Emmanuelle Rochette; Nadège Rouel; Oussama Saidi, Catherine Sarret and Daniel Terral.
Funding
This study was supported by a grant from Ligue Contre le Cancer, Comité départemental du Puy-de-Dôme.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualisation, A.A.; E.R.; P.D.; and J.K.; data curation, A.A; E.R; B.P.; formal analysis, B.P.; funding acquisition, J.K.; investigation, A.A.; E.R.; V.G.; S.M.; C.D.; E.L.; E.D.; F.I.; C.P.; and P.G-M.; methodology, A.A. and E.R.; project administration, A.A. and E.R.; validation, A.A.; E.R.; B.P.; J.K.; and P.D.; writing—original draft, A.A. and E.R.; writing—review and editing, A.A.; E.R.; J.K. and P.D. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Approval was granted by the Comité de Protection des Personnes Sud-Est V (No. 21.02921.000036 cat 1).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Implications/contribution
This work highlights the metabolic dysfunction met in young adult survivors after exposure to long courses of chemotherapy for the treatment of lymphomas during a crucial development period. It underlines the importance of early interventions to develop long-term health-sustaining behaviours.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Armand, A., Rochette, E., Grèze, V. et al. Fitness and metabolic response to exercise in young adult survivors of childhood lymphoma. Support Care Cancer 31, 358 (2023). https://doi.org/10.1007/s00520-023-07812-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07812-5